Booking options
£99
£99
On-Demand course
3 hours 30 minutes
Intermediate level
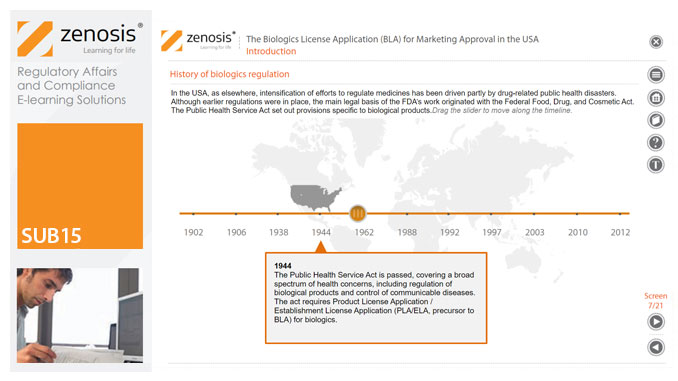
Biological products have come to dominate the market for medicines in recent decades. To introduce a biological product into interstate commerce in the USA, the sponsor of the product must gain FDA approval of a Biologics License Application (BLA). This enormous compilation of information and data is the culmination of years of effort and financial investment in the research and development of the product by the sponsor company. Biological products require manufacturing processes of greater complexity than those for small-molecule drugs, and control of the processes is critical to assurance of the products' safety, purity and potency. The processes and manufacturing establishments are subject to extensive inspection by the FDA before approval.
This module describes the requirements that must be met to obtain licensure of a biological product. Subjects covered include the regulatory context, the content and format of the BLA submission, the review process, and provisions for expedited development and review.
