Booking options
£99
£99
On-Demand course
3 hours
Intermediate level
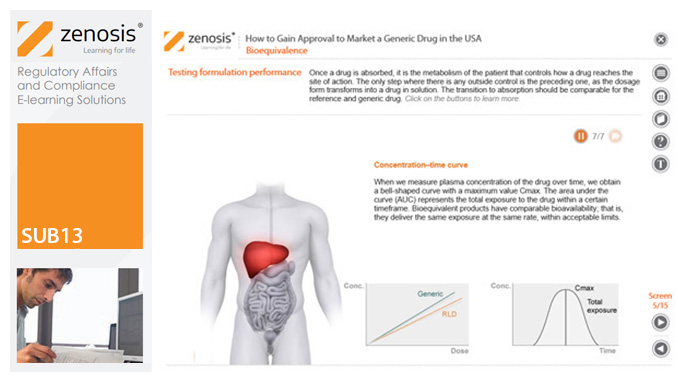
This module outlines the legislative and regulatory context for the development of generic drugs and describes the essential role of the Abbreviated New Drug Application (ANDA) in gaining marketing approval. The use of information in the ‘Orange Book’ is explained, as is the role of patent certification in the application. The importance of establishing bioequivalence between a generic and its reference product is emphasised. The module specifies the content and format requirements for an ANDA submission and describes the FDA’s review and approval process. An outline is given of the Generic Drug User Fee Amendments (GDUFA) and the law’s effects on industry players.
The module is up to date with the many final and draft publications, recently released by the FDA, that provide guidance for industry on applications for approval of generic drugs. It is also up to date with the provisions of the third authorisation of GDUFA, applicable in US fiscal years 2023 to 2027.
