- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
318 Nursing courses in Chorleywood
Cannulation Training – CPD Approved
By Lead Academy
Do you want to boost your medical career by learning about the cannulation process? This comprehensive cannulation course is ideal for you! You can learn about the whole venepuncture and cannulation procedure in this Venepuncture and Cannulation Training Course, as well as the safest methods for handling, flushing, and removing a cannula. This course will provide you with the necessary training to flourish in your profession, whether you are a novice or an experienced individual wishing to enhance your career in the healthcare industry. Course Highlights Designed by healthcare professionals Accredited by the CPD Certification Recognised and Valuable Certification Self-Paced Online Theoretical Learning High-Quality Study Materials Interactive practical training Practical Day Class Schedule – 1 Day Face-to-Face Training Venue Location London: Bank Studio, 23 Park Royal Road, NW10 7JH Date: 21st February, 2025 Date: 30th March, 2025 Date: 28th April, 2025 Date: 27th May, 2025 Date: 21st June, 2025 Time: 10:00 am – 05:00 pm Venue Location Birmingham: 83-85 Hagley Road, Birmingham, B16 8QG Date: 25th January, 2025 Date: 24th February, 2025 Date: 23rd March, 2025 Date: 21st April, 2025 Date: 26th May, 2025 Date: 26th June, 2025 Time: 10:00 am – 05:00 pm This extensive IV Cannulation Course covers the following credentials: Selection of veins for cannulation Arm and hand physiology and anatomy Types of cannula Process of cannulation Safety measures Proper insertion of the cannula Classroom-Based Cannulation Course Practical learning We offer comprehensive theoretical study materials and practical sessions for our cannulation training. As part of the practical training, you will receive practical dry lab demonstrations and hands-on experience to justify your role. Who is this Venepuncture and Cannulation Course for? This Venepuncture and Cannulation course is primarily aimed at: Health Visitors Healthcare professionals Nurses, midwives, pharmacists and doctors Freshers looking to begin their career in the healthcare sector Anyone looking to enhance their cannulation skills Aims and Learning Outcomes of this Venepuncture and Cannulation Training Course Know how to select the appropriate veins for cannulation Understand the anatomy and physiology of hands and arms Recognise the various sizes of a cannula Identify the risks concerning cannulation Know how to insert the cannula properly Recognise the safety precautions that should be maintained Gain knowledge about the dressing procedure Know how to safely flush and remove a cannula Learn how to securely dispose of all the sharps Learning how to perform Phlebotomy Trainingand Catheterisation Training can help you to provide better care to your patients. By being able to insert a catheter quickly and effectively, you can administer necessary treatments more efficiently, which can improve patient outcomes and reduce discomfort. Entry Requirements There are no academic entry requirements for this cannulation course, and it is open to students of all educational backgrounds. You need to join the Phlebotomy Training before attending the Cannulation Training. Assessment Method In this Venepuncture and Cannulation training, learners will be assessed via observation. That means during the practical training, you will be observed by the supervisor/trainer. Upon successful demonstration of blood sampling and blood draw, you will be awarded a CPD-accredited certificate accepted by thousands of professional bodies and government regulators in the UK and worldwide. Whether you are a fresher looking to kickstart your career in healthcare or practising healthcare looking to enhance your cannulation skills, this course will help you achieve your professional aspirations by all means. Course Curriculum 1. Cannulation Training - Course Introduction 2. What are Venepuncture and Cannulation 3. Anatomy and Physiology of the Circulatory System 4. Legal Aspects of Venepuncture (Phlebotomy) and Cannulation 5. Practitioner Requirements 6. Professional Competence and Accountability 7. Documentation and Record Keeping 8. Vicarious Liability 9. Consent 10. Influencing Factors during Venepuncture 11. Infection Prevention and Management 12. Haemolysis 13. Selecting Correct Equipment 14. Procedure of Venepuncture and Cannulation 15. Order of Draw 16. Peripheral Cannula and Venepuncture 17. Procedure Prior to Cannulation 18. During Cannulation 19. Cannula Size and Cannulation Aftercare 20. Removal of Cannulation 21. Complications 22. Cannulation Training - Conclusion Recognised Accreditation This course is accredited by Continuing Professional Development (CPD). CPD UK is globally recognised by employers, professional organisations, and academic institutions, thus a certificate from CPD Certification Service creates value towards your professional goal and achievement. CPD certificates are accepted by thousands of professional bodies and government regulators here in the UK and around the world. Many organisations look for employees with CPD requirements, which means, that by doing this course, you would be a potential candidate in your respective field. Certificate of Achievement On successful completion of the cannulation course, you will be eligible to obtain a CPD-accredited certificate of achievement as proof of your new skill. The certificate of achievement is an official credential that confirms that you successfully finished a course with Lead Academy.

Good Clinical Practice Auditing - Principles and Practice
By Research Quality Association
Course Information Our comprehensive course is used as a gateway to those stepping into the world of auditing clinical studies. Tailored for those already acquainted with Good Clinical Practice (GCP) and those transitioning from other audit disciplines, this programme stands as a pivotal guide. Pre-existing knowledge of GCP will significantly enhance your learning experience in auditing against these guidelines. How is this course run? Engage in immersive workshops providing hands-on practice with auditing techniques in a GCP context. Our seasoned tutors, boasting extensive audit experience, intertwine theory with practical insights drawn from their own professional journeys. What will I learn? A comprehensive understanding of the historical backdrop and objectives driving Good Clinical Practice, incorporating the latest industry developments Solid grounding in quality assurance activities aligned with regulatory standards Insight into potential pitfalls within clinical trials and the pivotal role of auditors in addressing these issues Clarity on the roles and responsibilities inherent to clinical trials auditing Exposure to a diverse range of audit techniques complemented by illustrative examples and supportive documents A nuanced understanding of regulatory inspectors' activities Expanded professional networks to propel your auditing career forward. Benefits include: A clear understanding of the role of the auditor under Good Clinical Practice improved audits Improved Good Clinical Practice compliance for your clinical trials. This course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Examine particular aspects of Good Clinical Practice. Tutors Tutors will be comprised of (click the photos for biographies): Rosemarie Corrigan EVP Global Quality, Worldwide Clinical Trials Cathy Dove Director and Owner, Dove Quality Solutions Julie Kelly Associate Director, Clinical Quality Assurance, Corcept Therapeutics Susana Tavares Director of Research Quality Assurance, - Programme Please note timings may be subject to alteration. Day 1 12:30 Registration 13:00 Welcome and Objectives for the first day of the course 13:30 Laying the Foundations Introduction to the clinical development process, the concepts of quality assurance, quality control and audit. 14:30 Break 15:00 Patient Protection Requirements for informed consent and ethics committee. Access to source documentation. Including a patient protection exercise. 16:05 Workshop 1 - Case Study on Informed Consent 16:45 End of Day Questions and Answers 17:00 Close of Day Day 2 08:50 Questions and Answers from Day 1 09:00 Effective Site Audits The procedures involved in selecting and setting up audits at investigator sites. 09:40 Workshop 1 - Planning the Effective Audit 10:30 Break 10:45 Source Data Verification The need for and purpose of verifying data. 11:25 Workshop 2 - Source Data Verification 12:30 Lunch 13:30 IMP Management The requirements surrounding the distribution of investigational medicinal products. Accountability from release to destruction. 14:15 Critical Document Audits The conduct of other study specific audits including protocols, databases and reports. 15:00 Break 15:15 Non-compliance Determining the acceptability of data. 16:00 Fraud - Fact or Fiction? How to identify fraud and its consequences 16:45 End of Day Questions and Answers 17:00 Close of Day Day 3 08:50 Questions and Answers from Days 1 and 2 09:00 Auditing Third Parties A review of audits of contract research organisations. 10:00 System Audits The concept of auditing processes across many clinical trials, including a practical exercise in process mapping. 10:45 Break 11:00 Workshop 3 - Process Mapping 11:45 Effective Audits Where theory meets reality. 12:30 Lunch 13:20 Audit Reports - Closing the Loop An examination of the processes which follow the evidence gathering phase of the audit. 14:20 Workshop 4 - Audit Reports Audit reports, corrective and preventive action. 15:00 Break 15:10 Regulatory Inspection Auditors and regulatory inspections -how the QA team can help the organisation to perform during a regulatory inspection. 15:55 Final Questions and Answers 16:10 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 17 Points Development Level Develop

GA Level 4 Award in Epilepsy and Buccal Midazolam Instruction
By Guardian Angels Training
Gain advanced knowledge and practical skills in instructing buccal midazolam administration for epilepsy with our Level 4 Award course.

Foundations of Immunisation and Vaccines
By Guardian Angels Training
Gain a comprehensive understanding of immunisation and vaccines with our "Foundations of Immunisation and Vaccines" course. Designed for healthcare professionals, public health workers, and individuals interested in immunisation, this course covers vaccine development, administration, safety, and the role of vaccines in preventing infectious diseases.
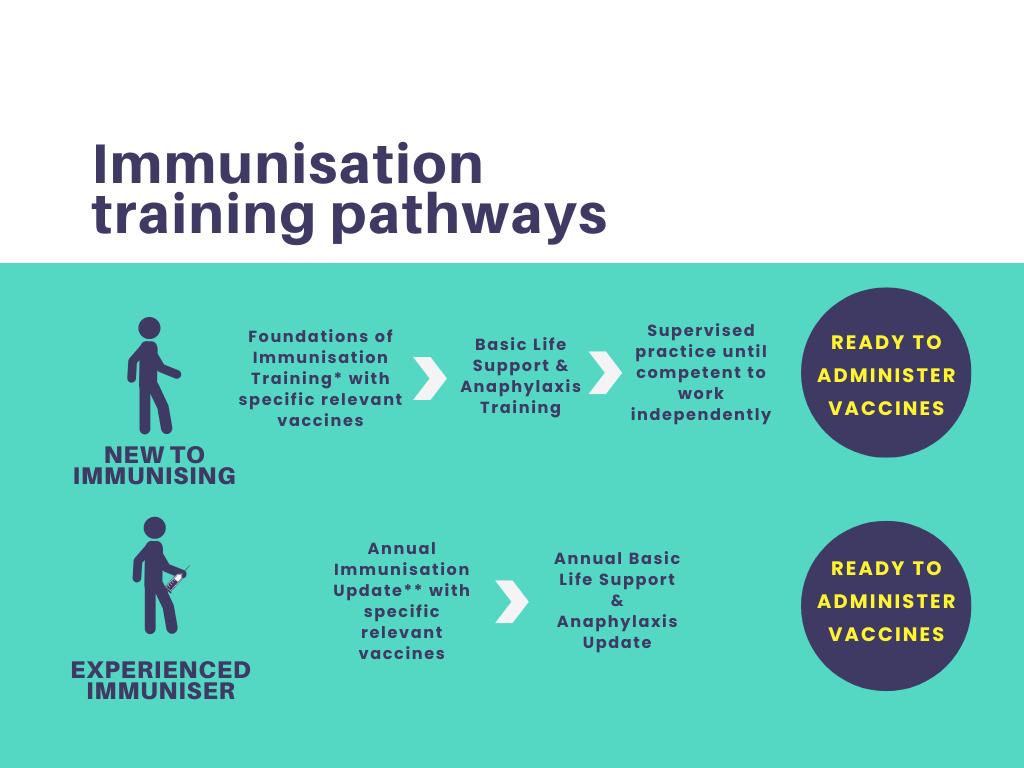
Working with Children and the Foundations of PBS
By Guardian Angels Training
Enhance your skills in promoting positive behavior and creating supportive environments for children with our PBS course. Evidence-based practices and collaboration emphasised.

Promoting Best Practice in Venepuncture Instruction
By Guardian Angels Training
Enhance your venepuncture skills with our comprehensive course. Learn evidence-based techniques, infection prevention measures, patient-centered communication, and ethical considerations to ensure safe and effective practices.
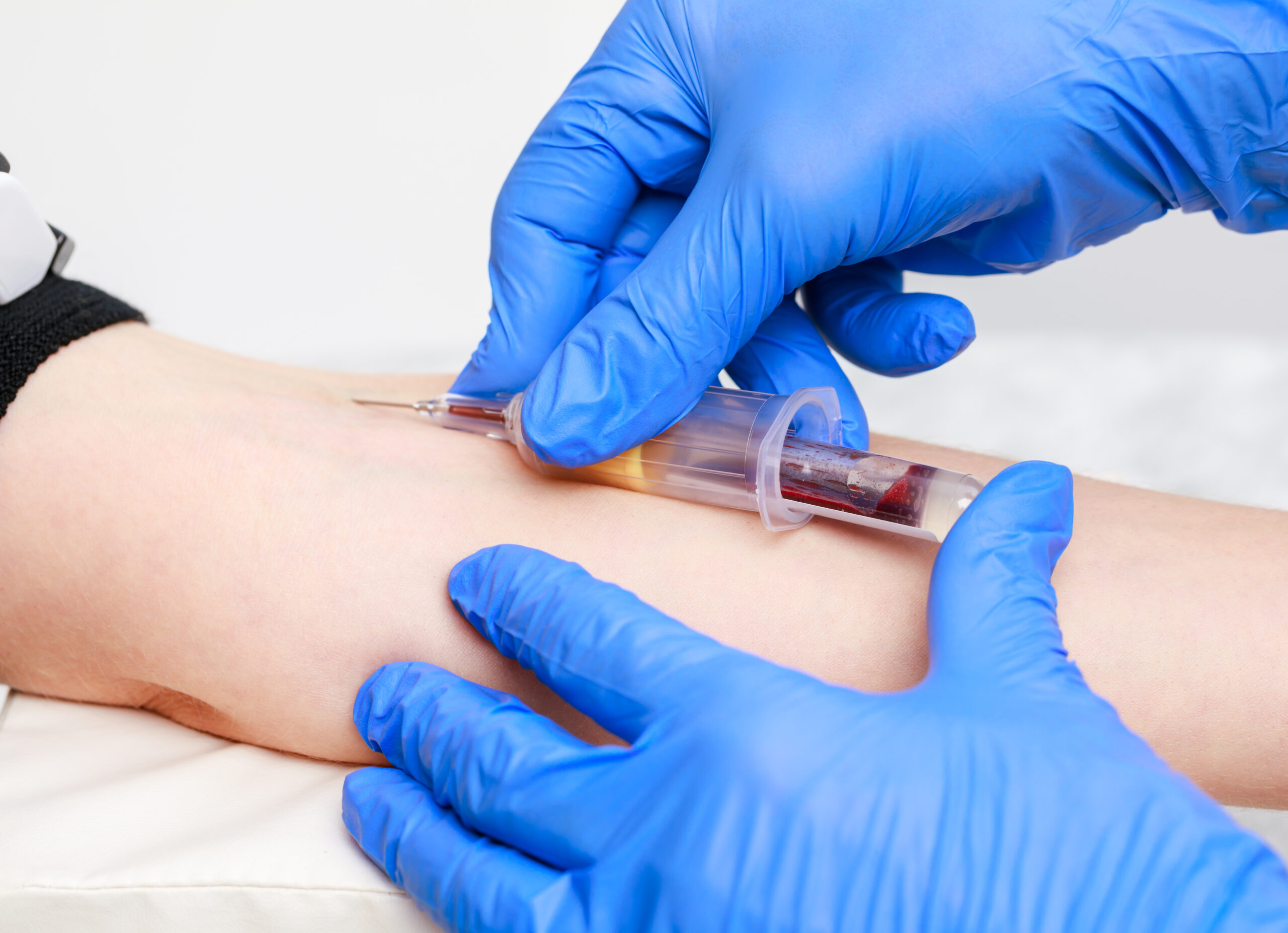
People Handling Instructor and Assessor
By Guardian Angels Training
Gain certification as a safe people handling instructor and assessor with our comprehensive course. Equip yourself with the necessary skills and knowledge to effectively train and assess others in safe manual handling techniques.

Promoting Best Practice in Basic Life Support Instruction
By Guardian Angels Training
Learn to teach basic life support effectively with our "Promoting Best Practice in Basic Life Support Instruction" course. Ideal for healthcare professionals, educators, and individuals interested in life-saving interventions.

Promoting Best Practice in Teaching and Assessing Medicines Management (Medication Train the Trainer)
By Guardian Angels Training
"Empower educators and healthcare professionals with evidence-based teaching strategies and practical assessment methods through our 'Promoting Best Practice in Teaching and Assessing Medicines Management' course. Ensure safe and competent medication administration practices among healthcare learners. Enroll now."
Promoting Best Practice in Asthma and Anaphylaxis Training (Train the Trainer)
By Guardian Angels Training
Gain comprehensive knowledge and practical skills in asthma and anaphylaxis management with our evidence-based training course. Ideal for healthcare professionals, educators, and patient care providers. Optimise patient outcomes with effective emergency response protocols, prevention strategies, and patient education.
Search By Location
- Nursing Courses in London
- Nursing Courses in Birmingham
- Nursing Courses in Glasgow
- Nursing Courses in Liverpool
- Nursing Courses in Bristol
- Nursing Courses in Manchester
- Nursing Courses in Sheffield
- Nursing Courses in Leeds
- Nursing Courses in Edinburgh
- Nursing Courses in Leicester
- Nursing Courses in Coventry
- Nursing Courses in Bradford
- Nursing Courses in Cardiff
- Nursing Courses in Belfast
- Nursing Courses in Nottingham