- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
8637 Courses
An Understanding of Diabetes, PoC Blood Glucose Monitoring, and the Administration of Insulin
By Guardian Angels Training
Gain comprehensive knowledge and practical skills for effective diabetes management with our course on blood glucose monitoring and insulin administration. Ideal for healthcare professionals.

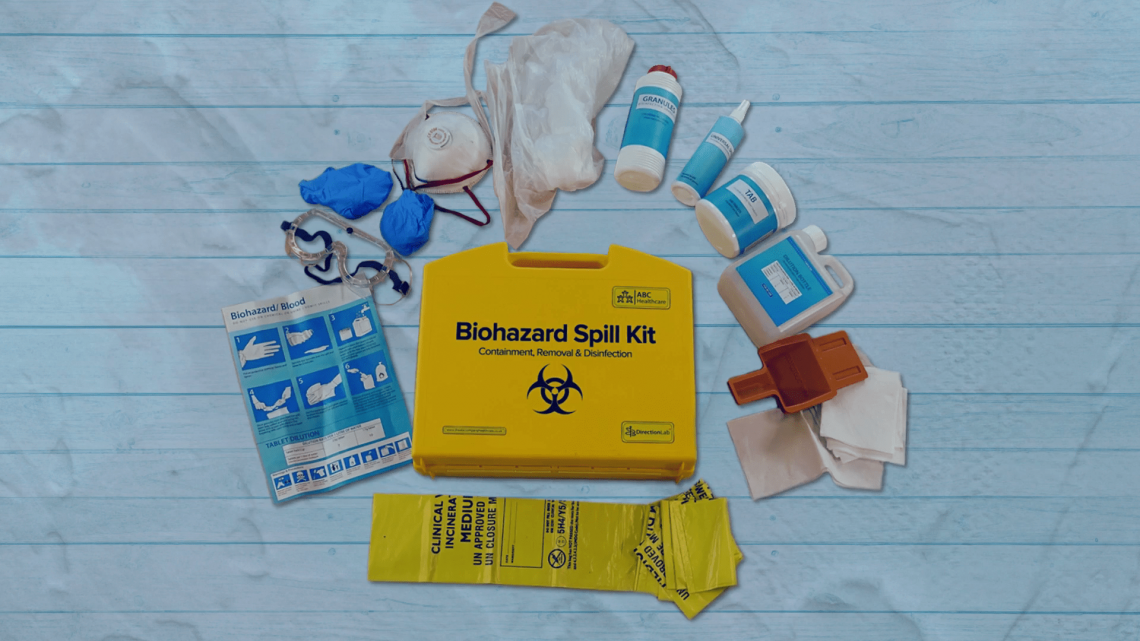
Biohazard Safety: Managing Blood and Body Fluid Spillages Instructor
By Guardian Angels Training
Gain expertise in biohazard safety with our "Biohazard Safety: Managing Blood and Body Fluid Spillages Instructor Training" course. Ideal for healthcare professionals, lab staff, and emergency responders.
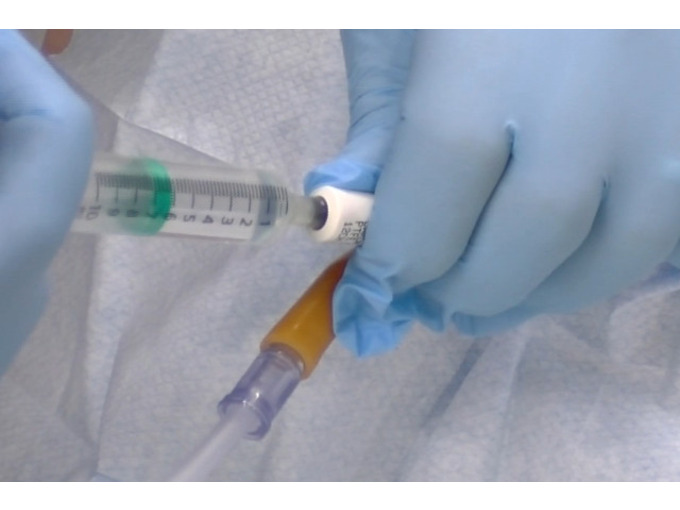
An Understanding of Male Catheterisation
By Guardian Angels Training
Gain comprehensive knowledge and practical skills for safe and effective male catheterisation with our course. Learn techniques, considerations, and patient care for addressing urinary retention, bladder dysfunction, and monitoring urinary output.
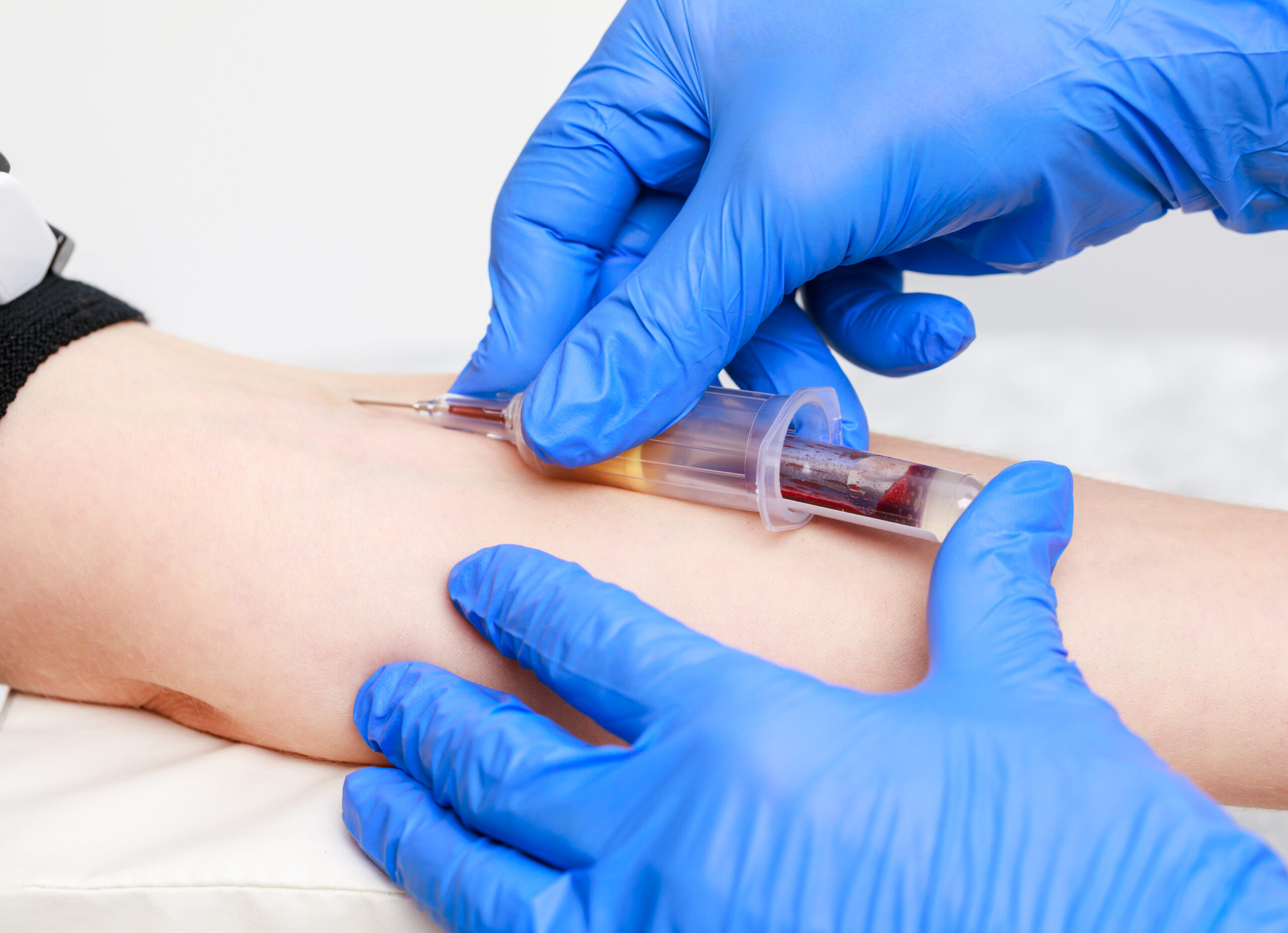
An Understanding of Venepuncture (Adult or Child as applicable)
By Guardian Angels Training
Gain comprehensive knowledge and practical skills for safe and effective venepuncture procedures in adult patients with our "Understanding Adult Venepuncture Techniques" course. Perfect for healthcare professionals seeking to confidently perform venepuncture with accuracy and patient comfort.
An Understanding of Physiological Observations
By Guardian Angels Training
Gain essential knowledge and practical skills to accurately monitor and interpret physiological parameters in patients with our "An Understanding of Physiological Observations" course. Learn to conduct and interpret various observations to support informed clinical decision-making.
An Understanding of Tracheostomy Care
By Guardian Angels Training
Gain comprehensive knowledge and practical skills to provide safe and effective care for tracheostomy patients with our "Understanding Tracheostomy Care" course. Learn the management and maintenance of surgical openings in the trachea to facilitate breathing.
Recognising and Responding to Acutely Unwell Individuals
By Guardian Angels Training
Gain the knowledge and skills to identify acute illness in patients with our "Recognising and Responding to Acutely Unwell Individuals" course. Improve patient outcomes and prevent deterioration in various healthcare settings. Enroll now.

An Understanding of Nasogastric Tube (NGT) Insertion and Feeding Training
By Guardian Angels Training
Nasogastric tube training ensures healthcare professionals have the correct clinical knowledge and skills to provide excellent care and support for individuals who may require treatment via NG tube for nutrition, hydration or medication.

An Understanding of Enteral Tube Care and Feeding
By Guardian Angels Training
Gain comprehensive knowledge and practical skills in enteral tube care and feeding with our course. Learn about insertion techniques, maintenance, feeding methods, and addressing complications to ensure safe and effective patient care.
An Understanding of Physiological Observations
By Guardian Angels Training
Enhance patient care with our "Understanding Physiological Observations" course. Gain comprehensive knowledge and skills to accurately assess and interpret vital signs and other measurements.

Search By Location
- Nursing Courses in London
- Nursing Courses in Birmingham
- Nursing Courses in Glasgow
- Nursing Courses in Liverpool
- Nursing Courses in Bristol
- Nursing Courses in Manchester
- Nursing Courses in Sheffield
- Nursing Courses in Leeds
- Nursing Courses in Edinburgh
- Nursing Courses in Leicester
- Nursing Courses in Coventry
- Nursing Courses in Bradford
- Nursing Courses in Cardiff
- Nursing Courses in Belfast
- Nursing Courses in Nottingham