- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
Advance Dry Needling Course (London, England) November 2025
By CPD Today
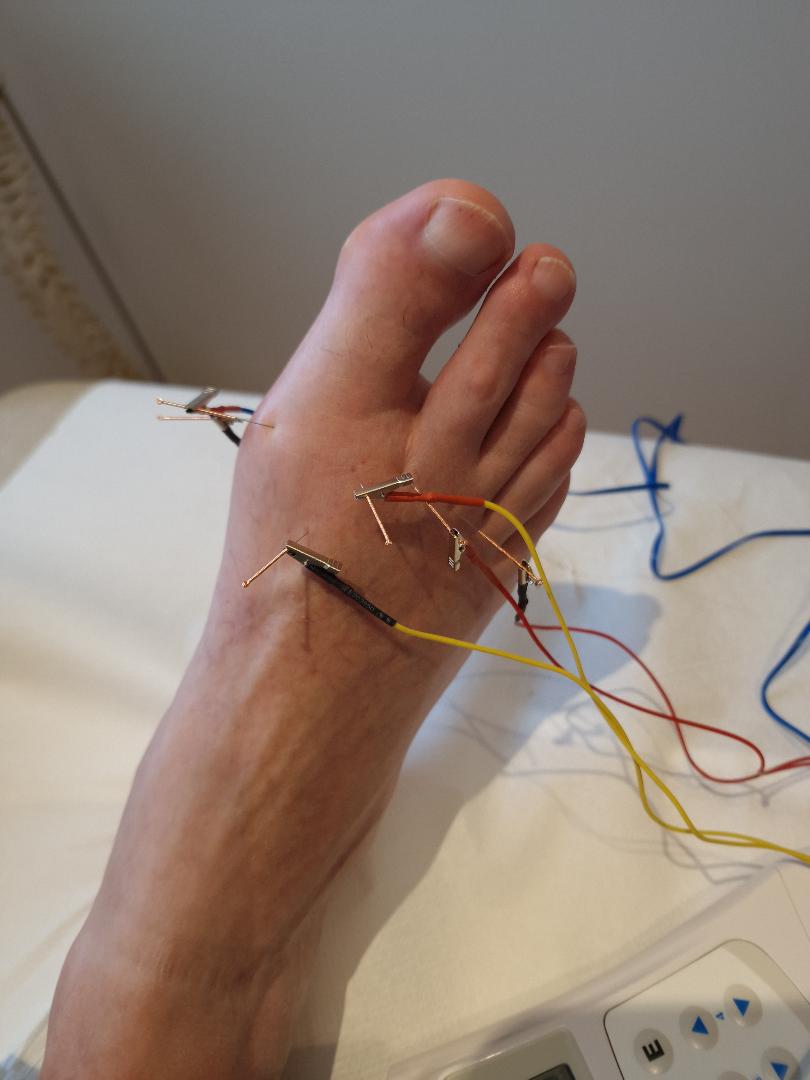
Dry needling course part 2, course is designed for all manual therapists including osteopaths, chiropractors, physiotherapist and sports therapists. To attend part 2 of the course you must have completed part 1.
GA Level 4 Award in Epilepsy and Buccal Midazolam Instruction
By Guardian Angels Training
Gain advanced knowledge and practical skills in instructing buccal midazolam administration for epilepsy with our Level 4 Award course.

Understanding Safe Clinical Practice Professional Development and Competence/Resilience/Mental Health and Wellbeing Interprofessional Communication Reflective Practice Health Promotion and Motivational Interviewing Clinical Skills and Chronic Disease-Asthma COPD/B12/Wound Care/Diabetes/Cardiovsacular/ECG's

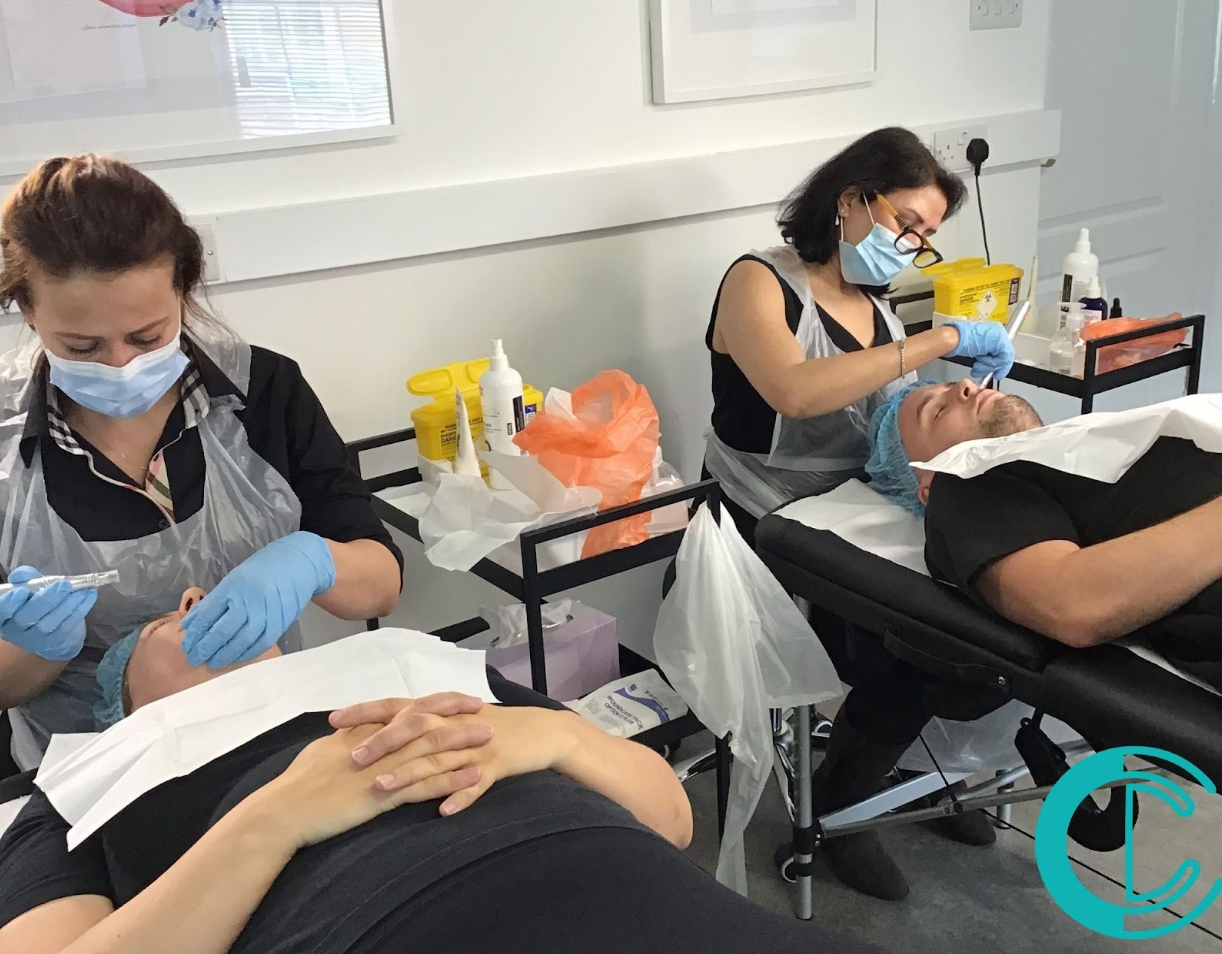
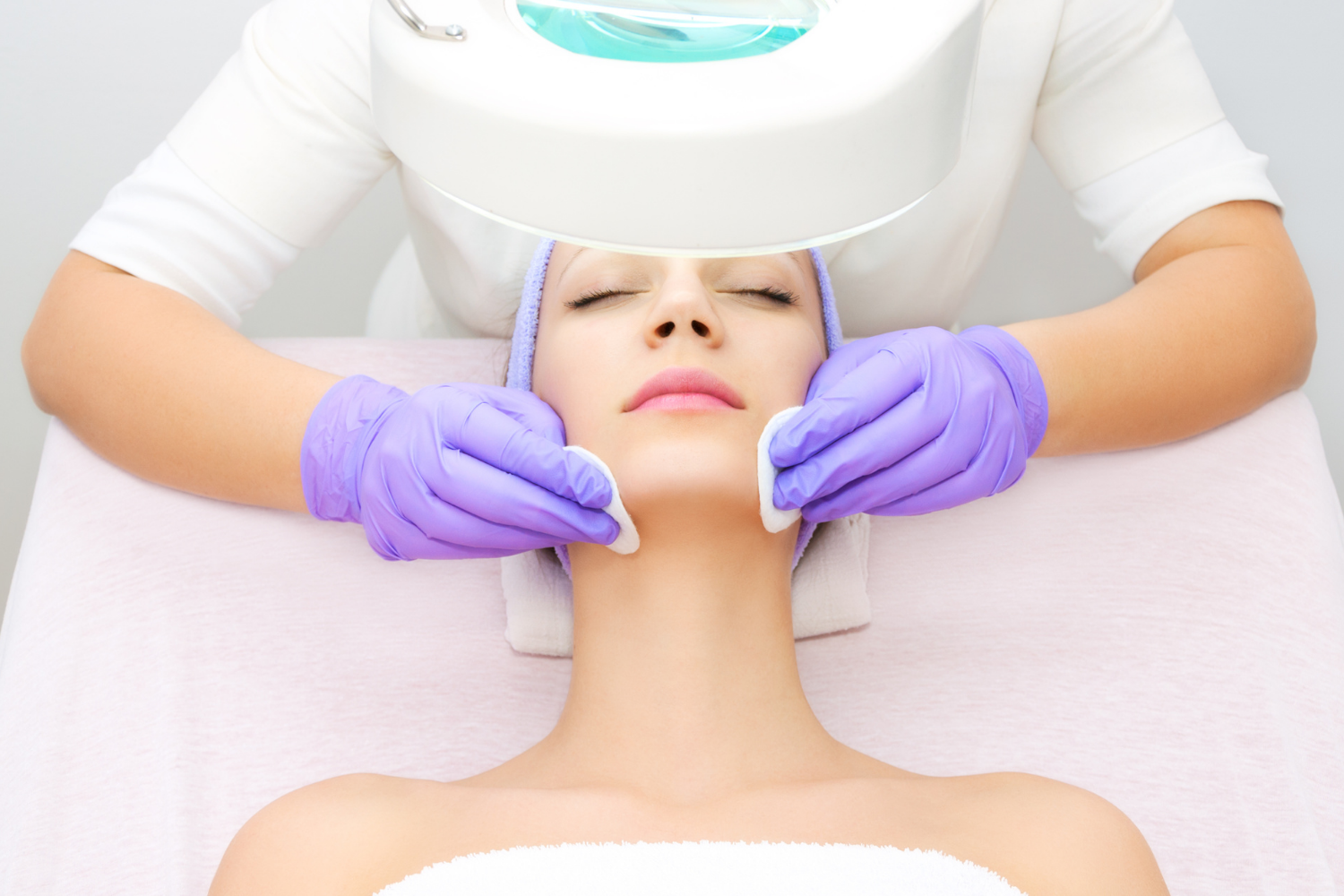
Comprehensive Aesthetic Specialist Training Package
By Cosmetic College
Take your beauty therapy skills to the next level with our Comprehensive Aesthetic Specialist Training Package. This all-inclusive package combines six of our most sought-after VTCT Level 4 courses, providing you with the knowledge and practical experience to offer a wide range of advanced aesthetic treatments. Our package includes the following courses: Level 4 Award in Skin Needling: Learn the art of skin rejuvenation with this microneedling course, which is ever growing in the industry. Level 4 Award in Dermaplaning: Learn the art of dermaplaning, a popular exfoliating treatment that removes dead skin cells and peach fuzz, leaving the skin smooth and radiant. Level 4 Award in High-Intensity Focused Ultrasound (HIFU): Gain the skills to offer HIFU treatments, a non-invasive procedure that tightens and lifts the skin, reducing the appearance of wrinkles and improving skin elasticity. Level 4 Certificate in Radio Frequency: Master the principles and practice of radio frequency therapy, a treatment that stimulates collagen production to reduce the appearance of fine lines and loose skin. Level 4 Chemical Skin Peels: Learn to perform skin peeling treatments, which can improve the appearance of the skin by reducing the visibility of scars, wrinkles, and hyperpigmentation. Level 4 in Ultrasound: Add ultrasound therapy to your repertoire, a treatment that can promote cellular renewal and repair, tone muscles, increase blood circulation, and improve skin care product penetration. Laser Hair Removal: Become a fully qualified laser aesthetic technician, capable of providing high-quality, safe, and effective laser hair removal treatments. Benefits of the Package: This comprehensive package is designed to equip you with a broad range of skills, making you a versatile and highly sought-after professional in the aesthetics industry. By completing these courses, you will not only expand your treatment offerings but also enhance your career prospects, whether you're looking to work in a salon, spa, or start your own business. What's Included: Each course in this package includes the VTCT Level 4 Qualification, all certificate and examination fees, refreshments, a complimentary treatment for your model, a professional treatment kit, membership discounts, and access to online community support groups. Flexible Payment Options: We understand that investing in your future is a big decision. That's why we offer flexible payment options, allowing you to spread your training costs over 3, 6, 9, or 12 months interest-free. Secure your place in our Comprehensive Aesthetic Specialist Training Package today with a minimal deposit. Join Us: Embark on a transformative journey with the Cosmetic College. Enhance your skills, boost your career, and become a highly qualified aesthetic specialist. Contact us today to learn more about our Comprehensive Aesthetic Specialist Training Package.
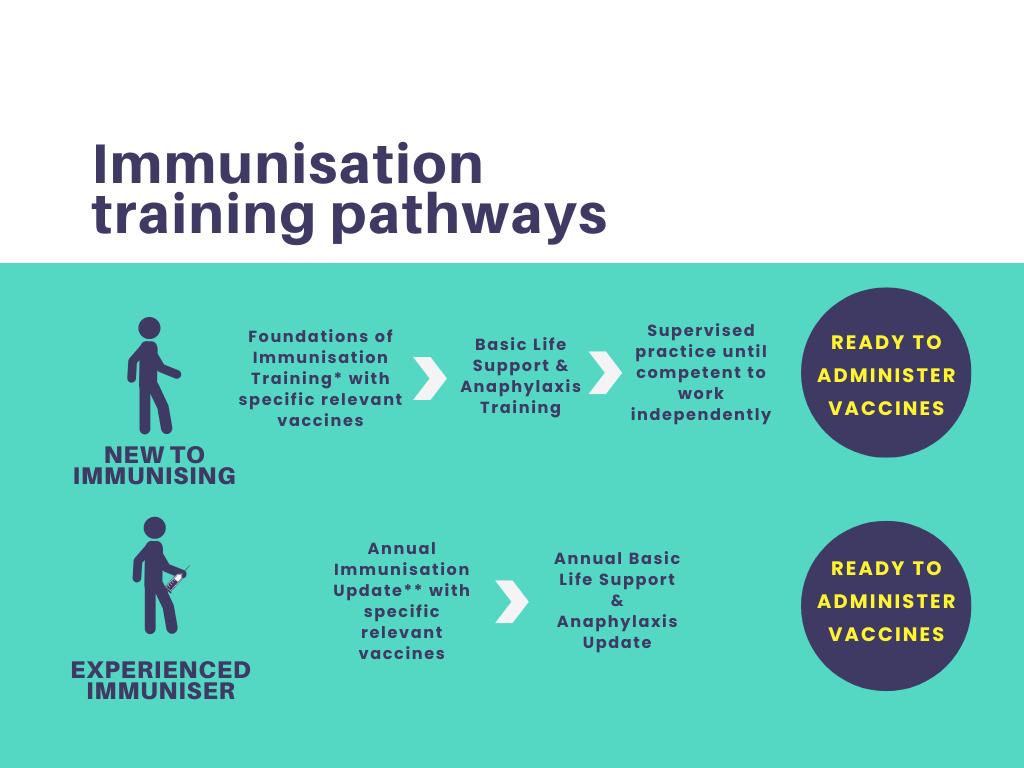
Foundations of Immunisation and Vaccines
By Guardian Angels Training
Gain a comprehensive understanding of immunisation and vaccines with our "Foundations of Immunisation and Vaccines" course. Designed for healthcare professionals, public health workers, and individuals interested in immunisation, this course covers vaccine development, administration, safety, and the role of vaccines in preventing infectious diseases.
Strapping and Taping Course October 2025
By CPD Today
Strapping & Taping Course covering Sports tape, k-tape (kinesio) & biomechanical tape. Perfect for Sports therapists osteopaths physiotherapists chiropractors

VTCT Levels 2, 3 & 4 Training Package: Your Journey to a Successful Career in Beauty Therapy
By Cosmetic College
Unlock your potential in the beauty industry with our Ultimate Value Training Package! This all-inclusive training package combines our highly sought-after VTCT Level 2 Facial & Skincare, VTCT NVQ Level 3 in Beauty Therapy, and VTCT Level 4 Laser Hair Removal & Skin Rejuvenation training courses. Invest in your future with this comprehensive package that will empower you with the knowledge and skills needed for a successful career in beauty therapy. What's Included: VTCT Level 2 Facial & Skincare Course Standalone Cost: 1,350 Learn the fundamentals of facial treatments and skincare Master techniques for various skin types and conditions Gain hands-on experience with industry-standard products and equipment VTCT NVQ Level 3 in Beauty Therapy Standalone Cost: 2,550 Expand your skills in advanced beauty treatments Delve into specialised techniques such as microdermabrasion and electrical treatments Enhance your client consultation and communication skills VTCT Level 4 Laser Hair Removal & Skin Rejuvenation Standalone Cost: 1,350 Master advanced techniques and cutting-edge beauty treatments Qualify in laser hair removal, light therapies, as well as aesthetic treatments Develop skills in managing a beauty therapy business Your Investment Ultimate Value Training Package - 4,230 (30% saving of 2,450!) Why Choose Our Ultimate Value Training Package? Comprehensive training: Our package covers a wide range of beauty therapy techniques, from foundational to advanced, ensuring a well-rounded education in the field. Expert instructors: Learn from experienced professionals who are passionate about sharing their knowledge and expertise. Flexible scheduling: Our courses are designed to accommodate your busy schedule with a variety of learning options, including full time, part-time and weekend classes. Hands-on experience: Gain valuable practical experience in our state-of-the-art training facilities. Industry-leading qualifications: Our courses are accredited by VTCT, ensuring you receive high-quality training that meets industry standards and is internationally recognised. Take the First Step Towards a Rewarding Career in Beauty Therapy Ready to embark on your journey to success in the beauty therapy industry? Our Ultimate Value Training Package is the perfect investment in your future. With comprehensive training, hands-on experience, and expert guidance, you'll be well on your way to a fulfilling and lucrative career. Don't miss out on this incredible offer! Enrol today and start building the foundation for a successful career in beauty therapy.
Fast Track To Aesthetics - Dermal filler training
By Cosmetic College
Are you looking to start a career in aesthetics but need the prerequisites for a traditional course? Look no further! Cosmetic College offers a fast-track pathway to becoming an accredited and insurable cosmetic injector. Our five day course is designed specifically for non-medics who want to pursue a career in aesthetics but need to meet the standard course prerequisites. Expert practitioners and educators deliver our course with years of industry experience. We prioritise safety and ensure that all learners will leave the system with the competency and knowledge necessary to perform injectables and aesthetic procedures with confidence. Before starting the course, you'll complete online pre-study and virtual lectures to give you a solid foundation in anatomy and physiology, health and safety, contraindications, complications, first aid, and infection control. Then, you'll spend 6-8 days of practical training, depending on your chosen course path, to hone your skills and get hands-on experience. Our fast-track pathway to injectables covers various topics, including microneedling, B12 dermaplaning, foundation lip dermal fillers, and more. To enrol in our fast-track course, you must be at least 21 years of age and have a good command of English. You should also be able to learn independently and have a strong desire to build a career in aesthetics. Invest in your future with Cosmetic College's fast-track pathway to injectables. With our comprehensive curriculum, expert instructors, and hands-on training, you'll be ready to start your aesthetic career. Take advantage of this opportunity to fast-track your aesthetic career. Enrol in our fast-track pathway to injectables today! Course Entry Requirements: Minimum age of 21 years Good command of English Be able to learn independently A strong desire to build a career in Aesthetics Course Pre-Study/Practical & Length: Online study and virtual lectures A series of online assessments Five days of practical training Course Agenda: Microneedling B12 Dermaplaning Foundation Lip Dermal Fillers Anatomy and Physiology Anaphylaxis Awareness Infection control Sharps and hazardous waste Complication management First aid Course Benefits Student Benefits Comprehensive Education: Our training program provides a comprehensive education on dermal fillers, equipping you with the knowledge and skills necessary to excel in the field of aesthetics. You will gain a deep understanding of facial anatomy, product selection, injection techniques, safety protocols, and patient assessment. Practical Hands-on Experience: We emphasise practical training, giving you ample opportunities to practice your skills under the guidance of experienced trainers. Working with live models will enhance your confidence and proficiency in performing dermal filler treatments. Industry-Standard Techniques: You will learn industry-standard techniques for administering dermal fillers, ensuring that you provide safe and effective treatments to your clients. This knowledge will enable you to achieve natural-looking results and meet the expectations of your clients. Personalised Guidance and Mentorship: Our trainers are highly experienced professionals who will provide personalised guidance and mentorship throughout the training. They will address your questions, offer constructive feedback, and help you refine your techniques, ensuring your continuous growth and improvement. Business and Marketing Support: In addition to the technical skills, we provide guidance on the business aspects of aesthetics. You will learn strategies for building and marketing your aesthetics practice, attracting clients, and maximizing your earning potential. This knowledge will empower you to establish a successful career in aesthetics. Client Benefits Expertise and Safety: Clients will have confidence knowing that you have received comprehensive training in dermal fillers. Your expertise will enable you to provide safe and high-quality treatments, minimising the risks and complications associated with the procedures. Natural and Desired Results: Through your training, you will be able to deliver natural and desired results to your clients. Your understanding of facial anatomy, product selection, and injection techniques will allow you to enhance their features, rejuvenate their appearance, and boost their self-confidence. Professionalism and Trust: By enrolling in our training program, you demonstrate your commitment to professionalism and continuous learning. Clients will trust in your skills and knowledge, knowing that you have received proper training from a reputable institution. Earning Potential Earning potential in the aesthetics industry can be significant. As a trained professional in dermal fillers, you can offer sought-after services to clients. The increasing demand for aesthetic treatments, combined with your expertise and ability to deliver exceptional results, can lead to a thriving client base and increased earning potential. The Fast Track To Aesthetics - Dermal Filler Training provides you with the foundation and skills necessary to capitalise on these opportunities and maximise your earning potential in the aesthetics field. Frequently Asked Questions Is this training suitable for beginners with no prior experience in aesthetics? Absolutely! Our Fast Track To Aesthetics program is designed to cater to beginners who are new to the field of aesthetics. We provide comprehensive training that covers the fundamentals of dermal fillers, ensuring that you gain the necessary knowledge and skills to start your journey in aesthetics. What topics are covered in the training program? Our training program covers a wide range of topics including facial anatomy, patient assessment, product selection, injection techniques, safety protocols, and client management. You will also receive hands-on training with live models to practice your skills under the guidance of experienced trainers. Are the trainers experienced in the field of aesthetics? Yes, our trainers are highly experienced professionals with extensive knowledge and practical experience in the field of aesthetics. They are dedicated to providing you with the guidance and support you need to excel in your training.

Strapping and Taping Course October 2025
By CPD Today
Strapping & Taping Course covering Sports tape, k-tape (kinesio) & biomechanical tape. Perfect for Sports therapists osteopaths physiotherapists chiropractors

Working with Children and the Foundations of PBS
By Guardian Angels Training
Enhance your skills in promoting positive behavior and creating supportive environments for children with our PBS course. Evidence-based practices and collaboration emphasised.

Search By Location
- Nursing Courses in London
- Nursing Courses in Birmingham
- Nursing Courses in Glasgow
- Nursing Courses in Liverpool
- Nursing Courses in Bristol
- Nursing Courses in Manchester
- Nursing Courses in Sheffield
- Nursing Courses in Leeds
- Nursing Courses in Edinburgh
- Nursing Courses in Leicester
- Nursing Courses in Coventry
- Nursing Courses in Bradford
- Nursing Courses in Cardiff
- Nursing Courses in Belfast
- Nursing Courses in Nottingham