- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
An Understanding of Suctioning
By Guardian Angels Training
Enhance respiratory care with our "Understanding Suctioning Techniques and Practices" course. Gain comprehensive knowledge and practical skills for safe and effective suctioning. Evidence-based practices, infection prevention, and patient-centred care emphasised.

Recognising and Responding to Acutely Unwell Individuals
By Guardian Angels Training
Gain the knowledge and skills to identify acute illness in patients with our "Recognising and Responding to Acutely Unwell Individuals" course. Improve patient outcomes and prevent deterioration in various healthcare settings. Enroll now.

Emergency Response for Care Homes
By Guardian Angels Training
The "Emergency Response for Care Homes" course is designed to provide care home staff with essential knowledge and skills to effectively respond to a wide range of emergency situations. This course aims to reinforce and update the participants' understanding of emergency protocols, procedures, and best practices, ensuring a safe and coordinated response in times of crisis.

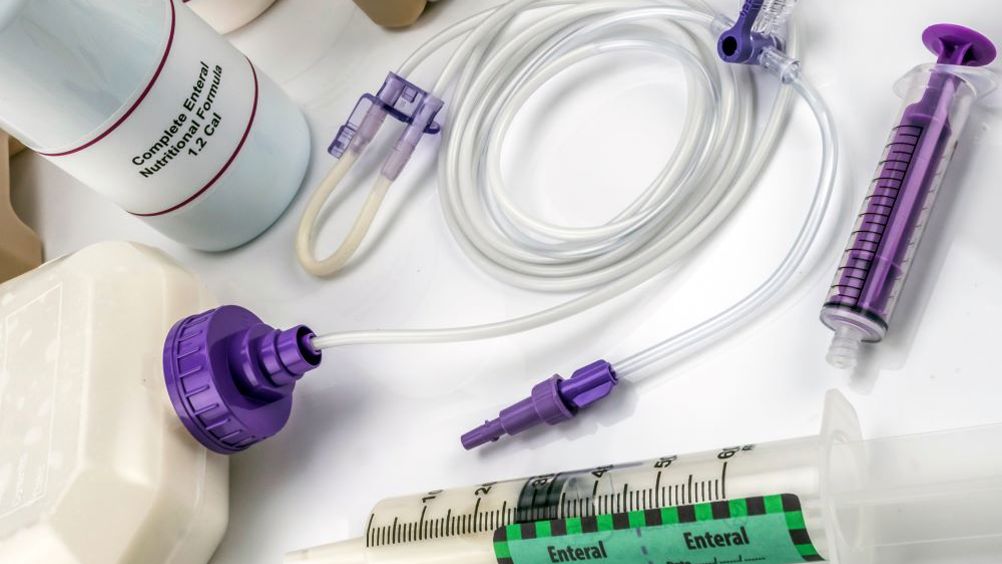
An Understanding of Enteral Tube Care and Feeding
By Guardian Angels Training
Gain comprehensive knowledge and practical skills in enteral tube care and feeding with our course. Learn about insertion techniques, maintenance, feeding methods, and addressing complications to ensure safe and effective patient care.
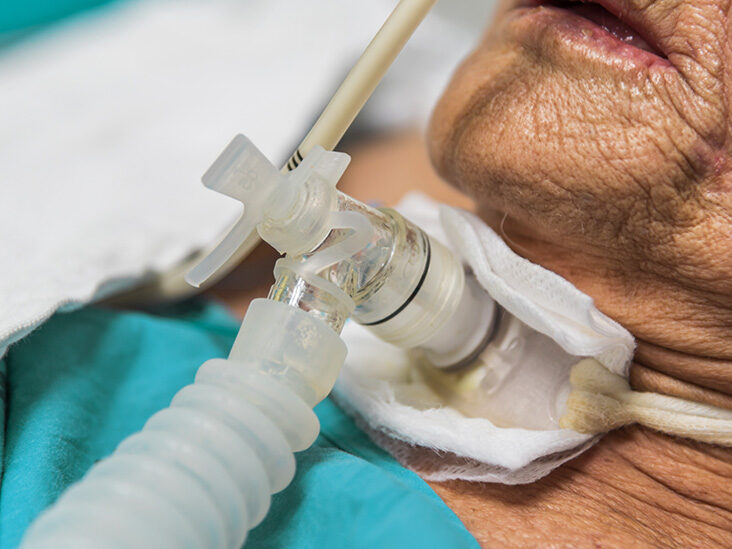
An Understanding of Tracheostomy Care
By Guardian Angels Training
Gain comprehensive knowledge and practical skills to provide safe and effective care for tracheostomy patients with our "Understanding Tracheostomy Care" course. Learn the management and maintenance of surgical openings in the trachea to facilitate breathing.
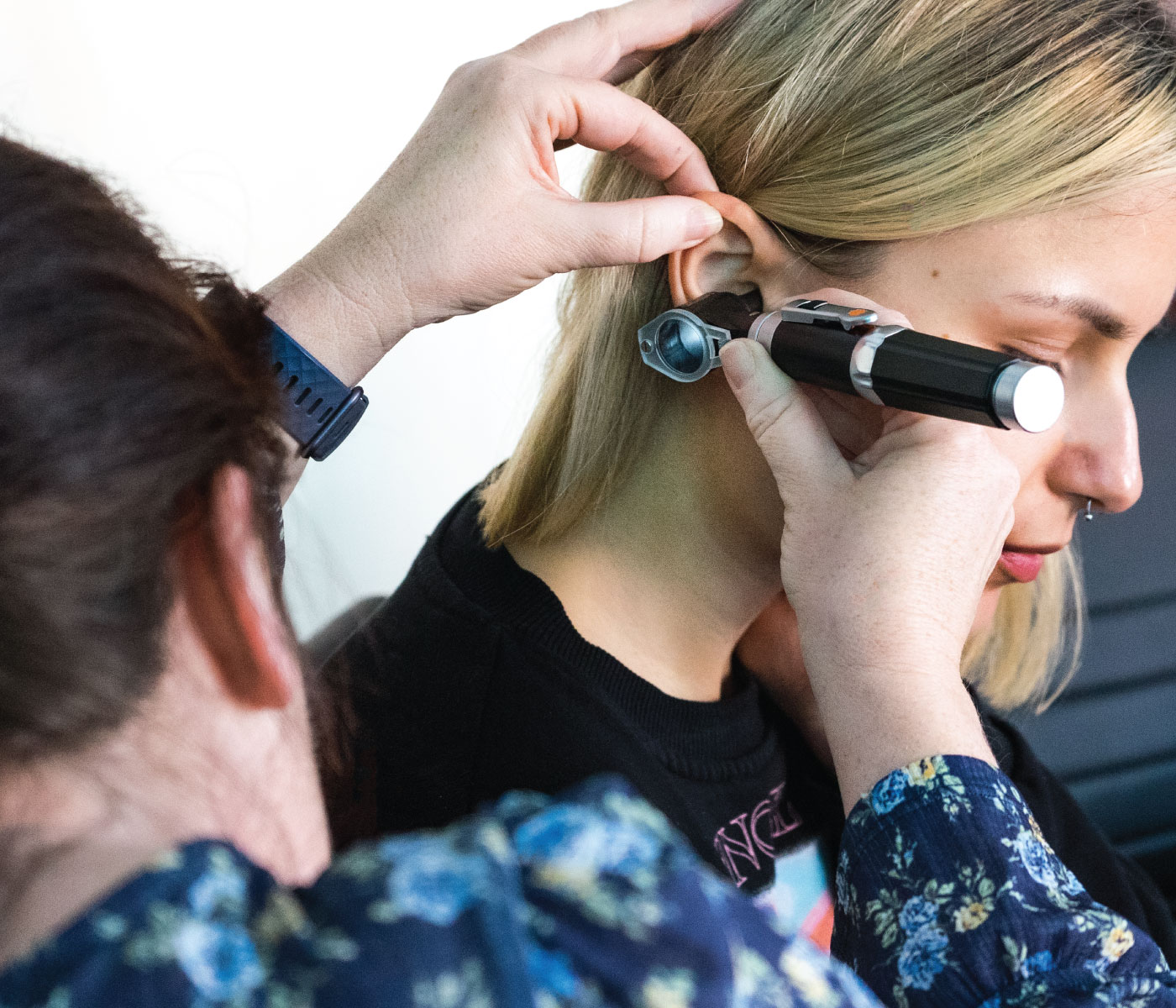
An Understanding of Ear Assessment and Care (including Ear Irrigation)
By Guardian Angels Training
Enhance your ear assessment and care skills with our comprehensive course on ear irrigation. Learn anatomy, assessment techniques, and safe practices for optimal ear health.
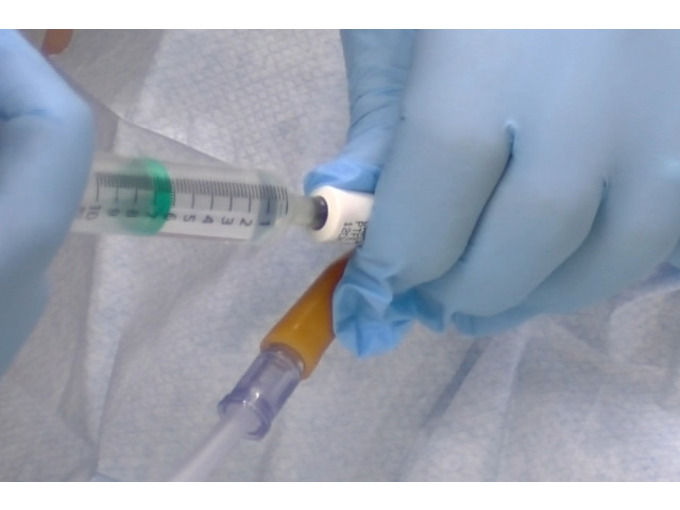
An Understanding of âSuprapubic Catheter Care
By Guardian Angels Training
Gain a comprehensive understanding of suprapubic catheters with our course. Learn about indications, insertion procedures, and ongoing care to ensure optimal outcomes for patients.
An Understanding of Physiological Observations
By Guardian Angels Training
Gain essential knowledge and practical skills to accurately monitor and interpret physiological parameters in patients with our "An Understanding of Physiological Observations" course. Learn to conduct and interpret various observations to support informed clinical decision-making.
An Understanding of Male Catheterisation
By Guardian Angels Training
Gain comprehensive knowledge and practical skills for safe and effective male catheterisation with our course. Learn techniques, considerations, and patient care for addressing urinary retention, bladder dysfunction, and monitoring urinary output.
An Understanding of Respiratory Care
By Guardian Angels Training
Enhance your respiratory care skills with our comprehensive course. Learn to assess, diagnose, and manage respiratory conditions for better patient outcomes.
Search By Location
- Guide Courses in London
- Guide Courses in Birmingham
- Guide Courses in Glasgow
- Guide Courses in Liverpool
- Guide Courses in Bristol
- Guide Courses in Manchester
- Guide Courses in Sheffield
- Guide Courses in Leeds
- Guide Courses in Edinburgh
- Guide Courses in Leicester
- Guide Courses in Coventry
- Guide Courses in Bradford
- Guide Courses in Cardiff
- Guide Courses in Belfast
- Guide Courses in Nottingham