- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
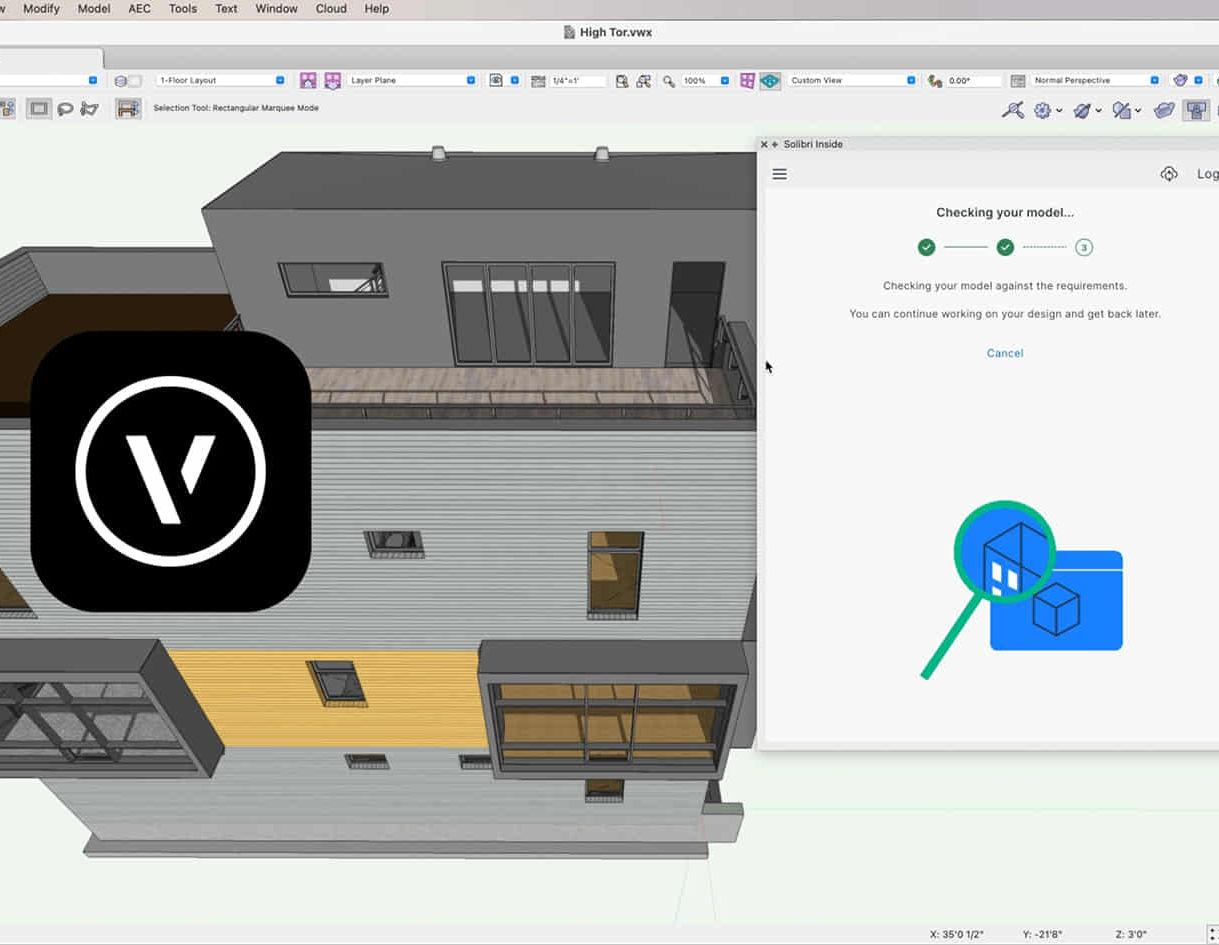
Vectorworks Intermediate Training Course
By ATL Autocad Training London
Who is this course for? Vectorworks Intermediate Training Course. This intermediate course empowers designers with Vectorworks skills, enhancing productivity and enabling the creation of intricate, high-quality designs. Design career with this essential training. Vectorworks courses cater to individuals of all skill levels. Click here for more info: Website Scheduling: 1-on-1 training, your schedule. Book any hour, Mon to Sat, 9 am - 7 pm. Call 02077202581 to reserve. Duration: 16 hours. Method: In-person sessions and live online. Comprehensive Course Outline Please note that the following is a general outline, and the specific topics covered during your training will be tailored to your student level, available time, and course preferences. Essential Foundations Navigating the Interface and User-Friendly Navigation Document Settings Adjustment and Template File Creation Effective File Navigation: Zooming, Panning, and Page Fitting Object Selection Techniques and Deletion Creating Basic Drawings with Precision Utilizing Object Snaps for Accurate Drawing Understanding the Object Info Palette Exploring Fundamental 2D Tools and Tool Modes Crafting Complex Lines and Shapes Project Organization Efficiently Organizing Your Drawings Using Classes Customizing Colors, Line Types, and Line Thickness Views Management: Saving and Editing Views Annotating and Printing Your Designs Adding Text and Annotations Incorporating Dimensions for Clarity Preparing and Printing Your Drawings Advanced Presentation Techniques Leveraging the Power of Sheets and Viewports Creating Standard and Cropped Viewports Annotating and Editing Viewports Exploring Viewport Display Overrides and Sheet Layer Printing Streamlining Workflows Between Viewports and Design Layers Efficiently Saving and Editing Views for Navigation Enhancement Importing DWG and File Formats Importing and Effectively Working with DWG Files Integrating Sketches and Photos into Your Drawings Enhancing Efficiency Creating and Editing Symbols Resource Browser: Management and Organization of Symbols Maximizing Productivity with Worksheets, Schedules, and Reports Custom Attribute Creation and Management (Hatches, Gradients, and Image Fills) Designing Custom Title Blocks Free 30-day Trial Vectorworks https://www.vectorworks.net/trial After completing this course, you will: Master Vectorworks: Gain proficiency in Vectorworks software, including its interface and essential design tools. Precise Drawing: Create accurate drawings with dimensions and object snaps. Project Management: Efficiently organize and manage design projects using classes and views. Clear Presentation: Annotate and dimension your drawings for professional presentations and printing. Advanced Presentation: Learn advanced presentation techniques, including sheets and viewports. File Integration: Work with DWG files, sketches, and photos to enhance your designs. Efficiency Boost: Discover time-saving workflows, symbol creation, and customization. Top Job Opportunities for Designers: Architectural Drafter Interior Designer Landscape Designer CAD Technician Graphic Designer Construction Estimator Product Designer Set Designer Event Planner Urban Planner These roles span various industries and offer career growth for Vectorworks-trained designers in fields like architecture, engineering, entertainment, and more. Tailored Vectorworks Training Our Vectorworks courses are customized to your needs, offering 1-2-1 sessions that adapt content to your expertise, experience, and project requirements. Whether in class, onsite, or via live online sessions, these bespoke courses ensure optimal learning outcomes. Course Overview Begin your journey into Vectorworks, unlocking the potential for digital 2D and 3D visualization in interior design, architecture, 3D modeling, and landscaping. Vectorworks, a user-friendly industry-standard software, is suitable for both PC and Mac users. Course Highlights Master proper drawing setup and utilize 2D drawing tools for professional plans and elevations. Navigate and leverage the built-in symbol library effectively. Transform 2D plans into fully rendered 3D perspectives with textures and lighting. Create a comprehensive project presentation print. (Note: This course does not cover complex curved shapes.) Tailored Training | Vectorworks Personalized One-on-One Guidance Flexible Learning Schedule Ongoing Post-Course Support Access to Extensive Learning Materials Recognition with a Certificate of Attendance Affordable Training Rates Assistance with Software Configuration Rewards for Referrals Special Group Training Discounts Convenient Hours to Suit Your Schedule Customized Courses for Your Animation Needs
Good Clinical Practice Auditing - Principles and Practice
By Research Quality Association
Course Information Our comprehensive course is used as a gateway to those stepping into the world of auditing clinical studies. Tailored for those already acquainted with Good Clinical Practice (GCP) and those transitioning from other audit disciplines, this programme stands as a pivotal guide. Pre-existing knowledge of GCP will significantly enhance your learning experience in auditing against these guidelines. How is this course run? Engage in immersive workshops providing hands-on practice with auditing techniques in a GCP context. Our seasoned tutors, boasting extensive audit experience, intertwine theory with practical insights drawn from their own professional journeys. What will I learn? A comprehensive understanding of the historical backdrop and objectives driving Good Clinical Practice, incorporating the latest industry developments Solid grounding in quality assurance activities aligned with regulatory standards Insight into potential pitfalls within clinical trials and the pivotal role of auditors in addressing these issues Clarity on the roles and responsibilities inherent to clinical trials auditing Exposure to a diverse range of audit techniques complemented by illustrative examples and supportive documents A nuanced understanding of regulatory inspectors' activities Expanded professional networks to propel your auditing career forward. Benefits include: A clear understanding of the role of the auditor under Good Clinical Practice improved audits Improved Good Clinical Practice compliance for your clinical trials. This course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Examine particular aspects of Good Clinical Practice. Tutors Tutors will be comprised of (click the photos for biographies): Rosemarie Corrigan EVP Global Quality, Worldwide Clinical Trials Cathy Dove Director and Owner, Dove Quality Solutions Julie Kelly Associate Director, Clinical Quality Assurance, Corcept Therapeutics Susana Tavares Director of Research Quality Assurance, - Programme Please note timings may be subject to alteration. Day 1 12:30 Registration 13:00 Welcome and Objectives for the first day of the course 13:30 Laying the Foundations Introduction to the clinical development process, the concepts of quality assurance, quality control and audit. 14:30 Break 15:00 Patient Protection Requirements for informed consent and ethics committee. Access to source documentation. Including a patient protection exercise. 16:05 Workshop 1 - Case Study on Informed Consent 16:45 End of Day Questions and Answers 17:00 Close of Day Day 2 08:50 Questions and Answers from Day 1 09:00 Effective Site Audits The procedures involved in selecting and setting up audits at investigator sites. 09:40 Workshop 1 - Planning the Effective Audit 10:30 Break 10:45 Source Data Verification The need for and purpose of verifying data. 11:25 Workshop 2 - Source Data Verification 12:30 Lunch 13:30 IMP Management The requirements surrounding the distribution of investigational medicinal products. Accountability from release to destruction. 14:15 Critical Document Audits The conduct of other study specific audits including protocols, databases and reports. 15:00 Break 15:15 Non-compliance Determining the acceptability of data. 16:00 Fraud - Fact or Fiction? How to identify fraud and its consequences 16:45 End of Day Questions and Answers 17:00 Close of Day Day 3 08:50 Questions and Answers from Days 1 and 2 09:00 Auditing Third Parties A review of audits of contract research organisations. 10:00 System Audits The concept of auditing processes across many clinical trials, including a practical exercise in process mapping. 10:45 Break 11:00 Workshop 3 - Process Mapping 11:45 Effective Audits Where theory meets reality. 12:30 Lunch 13:20 Audit Reports - Closing the Loop An examination of the processes which follow the evidence gathering phase of the audit. 14:20 Workshop 4 - Audit Reports Audit reports, corrective and preventive action. 15:00 Break 15:10 Regulatory Inspection Auditors and regulatory inspections -how the QA team can help the organisation to perform during a regulatory inspection. 15:55 Final Questions and Answers 16:10 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 17 Points Development Level Develop

Good Laboratory Practice for Study Directors, Principal Investigators, Study Staff and Management
By Research Quality Association
Course Information Embark on our GLP course offering extensive guidance and pragmatic support tailored for individuals serving as Study Directors or Principal Investigators overseeing non-clinical safety studies on pharmaceuticals, agricultural, and industrial chemicals within the realm of Good Laboratory Practice (GLP). This comprehensive programme extends its benefits to study staff and management operating in GLP-compliant environments. The course extensively covers the current OECD GLP Principles and UK GLP legislation, while also referencing international standards, regulations, and guidelines pertinent to the field. Benefits of this course: Practical help and guidance on the interpretation and application of GLP An opportunity to update your knowledge of GLP with the current interpretation of requirements Access to an experienced panel of speakers Information on how other organisations address GLP issues An opportunity to improve your understanding of the GLP requirements as they are applied in different situations. This course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Examine particular aspects of GLP Learn from the experience of others. Tutors Tutors will be comprised of (click the photos for biographies): Tim Stiles Consultant, Qualogy Ltd Tony Woodall Head of Quality Assurance, Alderley Analytical Gill Armour Study Monitor Team Leader, AstraZeneca Jane Elliston Senior Quality Assurance Auditor, Battelle UK Vanessa Grant -, - Jeanet Logsted CEO, Scantox Programme Please note timings may be subject to alteration. Day 1 09:00 Registration 09:15 Welcome and Introductions 09:35 Development of Good Laboratory Practice A review of the history of GLP, its current scope and application, with a synopsis of current European and international standards. 10:05 Roles and Responsibilities The responsibilities of study director, test facility, management and study staff in the conduct of a GLP study. 10:45 Break 11:00 The Roles and Responsibilities of the Study Director and Test Facility Management The role of the study director in the management and control of a study, as defined by GLP, and management's roles are explored. 11:45 Multi-site Studies What is a multi-site study and when should such concepts be applied on a study. The role of the study director and principal investigator in the planning, conduct and reporting of multi-site study are explored. 12:30 Study Plan (Protocols) GLP requirements for the preparation of a study plan, content, authorisation, amendments and deviations are discussed. 13:00 Lunch 13:45 Workshop 1 - The Study Plan Some practical problems with study plans and amendments explored. 14:45 Workshop 1 - Feedback 15:00 Standard Operating Procedures The control, content and authorisation of SOPs and the principles behind the practice. 15:30 Break 15:45 Workshop 2 - Practical Study Conduct Problems Dealing with practical problems encountered during the conduct of studies. 16:40 Workshop 2 - Feedback 17:15 Close of Day Day 2 09:00 Questions and Answers Discussion of issues raised by course delegates. 09:20 Quality Assurance The interactions between QA, management, study director and principal Investigator are discussed as is QAs role when conducting a multi-site study. 10:00 The Final Report The content of the final report and the role of those involved in its preparation and approval. Specific reporting requirements when conducting a multi-site study are also explained. 10:30 Break 10:45 Workshop 3 - Final Report Problems Practical problems of report preparation including compliance statements. 11:30 Workshop 3 - Feedback 12:00 Management of Raw Data and Records A view on how records and materials are managed and archived in compliance with GLP. 12:45 Lunch 13:30 Workshop 4 - Data and Sample Management Issues Dealing with data and sample management issues. 14:15 Workshop 4 - Feedback 14:45 Regulatory Inspection Government monitoring for compliance with Good Laboratory Practice. 15:15 Panel Session This panel session will address any outstanding issues raised by delegates. 15:45 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Develop

“Any good photography is a successful synthesis of technique and art.” – Andreas Feininger Discover the magic of Black & White Photography and learn some of the secrets the masters use to make stunning photographs. Explore the fundamentals of light and time and no longer be a slave to the Auto setting. Master the manual functions of your 35mm SLR film camera and unlock its true potential. Get to grips with the complete darkroom workflow from processing film to printing your own black & white photographs. Perfect for those with no experience or those looking for a full refresher. If you don’t have a 35mm SLR camera we have a limited number available for use during the course – please make your selection from the dropdown at time of booking. Week 1 Capture, technical notes, discussions, art context Understand the fundamentals of light and time in balancing an accurate exposure; No longer be a slave to the Auto setting! Learn how to utilise the Manual functions of your 35mm SLR camera including aperture, shutter speed, film speed and metering. Understand depth of field, composition and creative photo-making techniques; Appreciate photography within an art context – Begin to look at the work of other photographers Assignment: You will be given one roll of B&W film to be shot for the second week Week 2 Film processing Learn how to process B&W film by hand in Stills’ darkrooms. We will discuss different film types and chemistry, as well as best practice procedures for film handling. Appreciating photography within an art context-Sally Mann. Week 3 Contact Printing Gain an appreciation of general darkroom procedures;understand how to set up your enlarger and make a contact print using the traditional analogue method; learn how to make 8×10″ work prints using the single filter printing method. All paper and chemistry will be provided. Week 4 Darkroom printing This last session will allow participants to keep on printing with some one to one attention. We will also explore some basic dodging and burning and other printing techniques to get the most from your negatives and make a series of final prints. Courses are subject to minimum enrolment. Please register early, within five days of the start date, to reduce the likelihood of course cancellation. Please read our cancellation policy before booking. Students, anyone over the age of 65, and those in receipt of any form of benefits can claim the concessionary price, offering a 10% discount on the full course price. Valid proof of eligibility must be produced on the first day of the course. Please use the code CONCESSION when prompted at checkout. Stills uses ILFORD PHOTO chemicals on this course that can potentially pose a risk to pregnant and breast feeding women and asthmatics. We take every care to ensure good working practices and adequate ventilation in our darkrooms. If you feel you may be adversely affected, please visit the Health and Safety section of Ilford’s website for further information. General Guidance Notes for Pregnant and Breast Feeding Women and Asthmatics: From a risk assessment standpoint, provided all necessary control measures (such as good working practices, adequate ventilation, and the use of appropriate PPE) are in place then pregnant and breastfeeding women should be able to continue to work safely with photochemical products.Inhalation is the main route by which fumes and gases enter the body, making good ventilation a high priority. Exposure to irritant chemicals that would not affect most people may provoke an asthma attack in a person who already has asthma. For example, low levels of the gas sulphur dioxide can be produced by some ILFORD PHOTO processes. Most individuals would be unaffected but asthmatics may suffer adverse affects. ILFORD PHOTO products include no known human carcinogens, and no substances to which phrase R46 (May cause heritable genetic damage) or R64 (May cause harm to breastfed babies) applies. Most ILFORD PHOTO developers use hydroquinone, and their classification therefore includes R40 (Limited evidence of a carcinogenic effect) and R68 (Possible risk of irreversible effects). Some ILFORD PHOTO chemicals use boric acid or borates. These substances are classified as toxic for reproduction. As a result, the classification of some of the powder developers includes R60 (May impair fertility) and R61 (May cause harm to the unborn child).

Process Mapping and Standard Operating Procedure (SOP) Writing
By Research Quality Association
Course Information This course aims to empower you with the expertise to proficiently navigate process mapping and master the art of crafting Standard Operating Procedures (SOPs) within regulated environments. Whether you're engaged in activities that demand process improvement, continual enhancement, or SOP creation, this course offers invaluable insights tailored to your needs. It caters to individuals tasked with managing, documenting, and implementing processes and SOPs, irrespective of prior experience or skills. Our curriculum does not focus on specific software or approaches, focusing instead on fundamental principles and adaptable concepts applicable across diverse organisational landscapes. Benefits include: Hands-on experience in mapping processes An understanding of how process maps can be used to define, communicate and continually improve complex processes An understanding of best practice for SOPs using of process maps/process flow diagrams and process mind maps to complement text An insight into the preparation of concise and user friendly SOPs. This course is structured to encourage delegates to: Discuss and develop ideas Develop a practical approach for creating process maps and writing SOPs Understand how to use process maps in SOPs effectively Discuss how process mapping for preparing SOPs can be applied to process improvement for SOPs. Is this course for you? The course is designed for all those with responsibility for managing, documenting and implementing processes and SOPs. It assumes no prior experience or skills. The course does not recommend any specific software or approach, but explores the principles and ideas that can be applied in any organisation. What will you learn? Hands-on experience in mapping processes An understanding of how process maps can be used to define, communicate and continually improve complex processes An understanding of best practice for SOPs using of process maps/process flow diagrams and process mind maps to complement text An insight into the preparation of concise and user friendly SOPs. By the end of the course you will be able to: Create process maps and write clearer more concise SOPs Understand how to use process maps in SOPs effectively Understand how process mapping can be applied to process improvement and better SOPs. Tutors Tutors will be comprised of (click the photos for biographies): Laura Brown Director, Laura Brown Training and Development David Butler VP of Quality, Resolian Programme Please note timings may be subject to alteration. Day 1 09:00 Welcome, Introductions and Course Objectives Why Companies manage by process. 10:00 Exercise 1 - First Steps Mechanisms for process mapping, levels at which it can be applied, and the roles and responsibilities of process owners. 10:30 Exercise 1 - Feedback 10:45 Break 11:00 Mapping Processes The stages involved in mapping processes including how to construct a SIPOC chart. 11:45 Exercise 2 - Mapping A Process A first opportunity to practice your new process mapping skills. 12:30 Exercise 2 - Feedback 13:00 Lunch 14:00 Exercise 3 - Discussion of Options for Format, Structure and Layout What Makes A Good SOP? Discussion of options for format, structure, level of detail of SOPs and the use of process maps for SOP writing. A case study example of a good SOP which uses a flow chart/process map. 14:45 Exercise 3 - Feedback 15:00 Break 15:15 A Case Study example of a good SOP that uses a flow chart / process map 15:30 Exercise 4 Discussion of the content of an SOP using a Process Map 15:35 Exercise 4 - Feedback 15:45 Key Writing Considerations 16:15 Exercise 5 16:45 Exercise 5 - Feedback 17:00 Close of Day Day 2 09:00 Review of Day 1 09:15 Using Metrics Selecting and using metrics to monitor and improve processes. 10:00 Exercise 6 - Practice in Process Mapping Creating Process Maps from multi-source information. 11:00 Break 11:15 Exercise 6 - Feedback 11:45 Common Pitfalls Common problems and tips for good Process Mapping. 12:15 Demonstration of Process Mapping on a PC 12:45 Lunch 13:30 Exercise 7 - Mind Mapping 14:30 Exercise 7 - Feedback 15:00 Break 15:15 Exercise 8 - Using Process Maps to Write an SOP Applying your process mapping skills as part of writing an SOP 15:30 Exercise 8 - Feedback 15:45 Course Review and Follow-up 16:00 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. Development Level Learn

A NEW one-day watercolour painting workshop for beginners run by local artist and printmaker Lou Davis in our Edinburgh City Centre workshop space. Join artist Lou Davis for a whole day learning to paint in beautiful fluid watercolours. Lou uses watercolour to create simple and approachable patterned and abstract pieces that allow you to get acquainted with this medium in a way that helps you focus, shut out the outside world and simply create. In this workshop, you’ll learn everything you need to get started painting beautiful and relaxing projects using watercolour. We’ll cover a number of projects throughout the day, that will take you through the basics of watercolour from colour mixing and making different brush strokes, learning about paper types and water control, through to mixed media techniques: adding textures, working with masking fluid and embellishing your pieces with pens and metallic paints. This workshop is perfect if you’re new to watercolour and offers a really beautiful way to explore the medium, it’s also good if you’re experienced in watercolour and are interested in using your painting and drawing time to relax and de-stress. All materials for this workshop will be provided, and the price includes morning and afternoon refreshments from local suppliers. You are welcome to bring your own lunch, or there are several cafés and takeaway options nearby. Earth conscious credentials We are mindful of the effect we have on the environment, and do our best to minimise our impact. You will take the lovely watercolours you have created home with you on the day, and workshop correspondence and notes are sent to you electronically. Favourite feedback We love to hear what you think about our workshops. Here's a favourite review for previous watercolour painting workshop with Lou. A totally peaceful and mindful three hours spent being guided and inspired by Lou…Just going with the flow. Enjoying the process. And being quite surprised by the results. Lynn is a wonderful host at The Arienas Collective…Thanks Lynn and Lou for a mindful morning. [Judith] More information The Arienas Collective workshops, courses and events are run from a welcoming and supportive environment within a Georgian mews home in Edinburgh’s City Centre New Town. We invite you to join us, leave any day-to-day worries behind and immerse yourself in being creative. Spaces on our workshops and courses are limited so that our creative team of artists and makers can offer you the hands-on help and support you need to make the most of your time with us. Our taster and introductory workshops focus on teaching creative skills and methods you can continue practising at home, using affordable materials and equipment. Where more expensive equipment is required we will mention this in the workshop description. All the equipment and materials you need are included in the price, unless stated otherwise. Light refreshments from local suppliers are provided. On full day workshops you are welcome to bring your own lunch to eat inside or out. There is also a wide range of independent sit-in or takeaway delis, cafés, and restaurants, plus a range of supermarkets all within a 5-10 minute walk. Accessibility The workshop space is on the first-floor of our Georgian mews home and unfortunately we do not have lift access. Blue badge holders can park outside our front door on the single yellow line by arrangement. Allergies The workshop space is in our open plan living space and is not nut free. Please let us know about any skin or food allergies before booking. We offer a dairy-free milk and gluten free snack alternative. Minimum number no refund policy To ensure we can always pay our creatives a fair fee, we require a minimum number of bookings for each workshop and for this reason bookings are non-refundable. Should we have to cancel due to low numbers, we will offer you the option to swap to an alternative date or workshop, or to request a refund. We do our very best to avoid cancellations. Since we often get late bookings, we keep our workshops on sale for as long as possible. We realise that some of you travel from afar, so if you book a space and have not yet received an email confirming your workshop is going ahead and need to make plans for your trip, please get in touch before booking any travel or accommodation so we can update you on booking numbers. Or check in before booking. Private workshop bookings Most of our workshops are also available to book for: private get-togethers with family and friends; themed wedding and birthday celebrations; corporate team building and well-being purposes; and in-store retail events for your customers. If you’d like to know more please Email Lynn. Age restrictions Our workshops are planned with adults in mind. Many are suitable for teenagers, but some may require under 16s to be accompanied for insurance purposes. Please check with us before booking.

Excel - intermediate (In-House)
By The In House Training Company
This one-day course focuses on issues such as writing formulas and accessing help while writing them, and taking formulas to the next level by nesting one inside another for a powerful formula result. It also looks at ways of analysing data with reports, summarised by varying criteria. A range of time-saving tips and tricks are shared. This course will help participants: Calculate with absolute reference Group worksheets Link to tables Use the function library effectively Get to grips with the logical IF function Use conditional formatting Create pivot table reports Use data validation Master the VLOOKUP function 1 Calculating with absolute reference The difference between a relative and absolute formula Changing a relative formula to an absolute Using $ signs to lock cells when copying formulas 2 Grouping worksheets Grouping sheets together Inputting data into multiple sheets Writing a 3D formula to sum tables across sheets 3 Linking to tables Linking to a source table Using paste link to link a table to another file Using edit links to manage linked tables 4 The function library Benefits of writing formulas in the function library Finding the right formula using insert function Outputting statistics with COUNTA and COUNTBLANK Counting criteria in a list with COUNTIFS 5 Logical IF Function Outputting results from tests Running multiple tests for multiple results The concept of outputting results from numbers 6 Conditional formatting Enabling text and numbers to standout Applying colour to data using rules Managing rules Copying rules with the format painter 7 View side by side Comparing two Excel tables together Comparing two sheets together in the same file 8 Pivot table reports Analysing data with pivot tables Managing a pivot table's layout Outputting statistical reports Controlling number formats Visualising reports with pivot charts Inserting slicers for filtering data 9 Data validation Restricting data input with data validation Speeding up data entry with data validation 10 VLOOKUP function Best practices for writing a VLOOKUP A false type lookup A true type lookup Enhance formula results with IFNA 11 Print options Getting the most from print Printing page titles across pages Scaling content for print

Implementing Good Clinical Laboratory Practice
By Research Quality Association
Course Information Join our comprehensive course, meticulously designed to equip individuals implementing Good Clinical Laboratory Practice (GCLP) within laboratories handling samples from clinical trials. Delve into the current regulatory landscape governing laboratory work supporting clinical trials, referencing key guidelines such as the ICH Guideline for Good Clinical Practice, the Clinical EU Trials Directive, relevant regulations, and leveraging insights from the RQA guidance document on GCLP. Is this course for you? This course is tailored for laboratory managers, analysts, investigators, trial coordinators, monitors, and auditors operating in diverse settings such as pharmaceutical company laboratories, central laboratories, contract research organisations, hospital laboratories, clinics, and investigator sites. This course will give you: Guidance on effectively interpreting and applying GCLP within the broader framework of Good Clinical Practice (GCP) Insight into the seamless integration of GCLP within clinical programmes (GCP) Practical strategies for implementing GCLP in the nuanced environment of clinical research laboratories The chance to update your knowledge with the latest interpretations and guidance on clinical laboratories by the UK Medicines and Healthcare Products Regulatory Agency (MHRA) Access to a seasoned panel of speakers with extensive expertise A unique opportunity to deepen your understanding of GCLP's application across diverse scenarios. Engage in: Lively discussions to foster ideas Problem-solving sessions targeting specific challenges Detailed exploration of specific aspects within the realms of GCP and GCLP. Tutors Tutors will be comprised of (click the photos for biographies): Vanessa Grant -, - Louise Handy Director, Handy Consulting Ltd Tim Stiles Consultant, Qualogy Ltd Programme Please note timings may be subject to alteration. Day 1 08:50 Registration 09:00 Welcome and Introduction 09:20 Good Clinical Practice and the Requirements of Good Clinical Laboratory Practice A review of Good Clinical Practice and its requirements for the laboratory analysis of samples from a trial. The thought processes behind the development of Good Clinical Laboratory Practice, its objective, scope, interpretation and application are explained. 10:00 Safety and Ethical Consideration Informed consent, confidentiality, expedited reporting, blinding and unblinding and serious breaches of the GCP are discussed. 10:40 Break 10:55 Organisation and Personnel Responsibilities within GCP and the Laboratory The responsibilities of key functions that should exist within a clinical laboratory including personnel records of training and competence are discussed. 11:30 Staff Training and Training Records Personnel records of training and competency assessments are discussed. 11:45 Laboratory Facilities, Equipment and Materials Suitable facility design, organisation and operation will be discussed. The calibration, validation and maintenance of equipment used in the conduct of sample analysis are examined, as are the suitability of materials and the identification and labelling of reagents and solutions. 12:30 Lunch 13:15 Workshop 1 - Facilities, Equipment and Responsibilities Some practical problems with regard to the facilities, equipment and responsibilities are explored. 13:45 Workshop 1 - Feedback 14:15 Computer Systems Validation Systems, including computerised systems, used in the analysis, collection and reporting of results should be appropriately tested, operated and controlled. What this means in practice is discussed. 14:45 Trial Protocols, Analytical Plans During this session we examine the purpose, content, control and change of these important documents. 15:30 Break 15:45 Workshop 2 - SOPs, Clinical Protocols, Analytical Plans and Validation The practicalities of managing and documenting the planning phase of analytical work on a trial are explored along with computerised system validation. 16:30 Workshop 2 - Feedback 17:00 Close of Day Day 2 09:00 Conduct of the Work and Quality Control Many of the issues that surround the conduct of sample collection, shipment, storage, analysis and management of Analytical Methods are discussed. This includes the quality control of the assay that may be employed and Quality Control checks. 10:00 Deviation Management The expectations around deviations and CAPA are discussed. 10:15 Workshop 3 - Conduct of the Work and Quality Control Practical work conduct and quality control issues are explored. 10:45 Break 11:00 Workshop 3 - Feedback 11:30 Source Data, Data Integrity, Records and Reports The creation and subsequent management of source data and records, data integrity, are discussed, together with the process of reporting analytical results. 12:10 Workshop 4 - Data, Records and Reports Practical problems with data, records and reports are investigated. 12:45 Lunch 13:30 Workshop 4 - Feedback 14:00 Quality Audit The requirements for and purpose of quality audits are discussed. The difference between quality audit and quality control are explained along with the role of the quality audit staff and their interaction with the analytical project managers, laboratory management and study staff. 14:40 Risk Management How should we assess risk and how can we use the process to assist in evaluation of audit findings. 15:15 Break 15:30 Regulatory Inspection The conduct of regulatory inspections and current expectations of the inspectors. Preparation for inspections and conduct during them will be discussed. 16:00 Panel Session This panel session will address any outstanding issues raised by the delegates. 16:15 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Develop

AutoCAD-Mac Introduction to Advanced Training Course
By ATL Autocad Training London
AutoCAD-Mac Introduction to Advanced Training Course This project-based course is tailored for AutoCAD Mac users, architecture and interior design students and professionals. Click here for more info: Website When Can I Book This Training Course? Personalized experience with 1-on-1 sessions designed to fit your unique needs. Available from Monday to Saturday, 9 am to 7 am Or Call to Book 02077202581. Duration of Training: 16 hours. Training Method: 1-on-1, providing the option for in-person sessions or live online training. Course Content Section 1: Introduction Course Overview Section 2: Basic Concepts Status Bar and The Units Drawing Commands Control Points - Vertex or Verteces Modifying Commands Snap Settings Drawing An Outline Section 3: Dimensions and Customization Adding Dimensions and Dimension Style Manager Importing Dimension Styles Customizing Shortcuts Section 4: Advanced Techniques Adding Walls Thickness with Offset Command Adding interior layout Cleaning Layout lines with Trim Section 5: Text and Styling Text Editing and Importing Text Styles Main File Formats in AutoCAD Section 6: Design Elements Creating Openings in the Floor Plan Adding Openings - Part 2 Adding Openings - Part 3 Drawing Doors & Windows Using Line Types & Linetype Scale Group and Ungroup Commands Using Blocks Using Blocks Library & Dynamic Blocks Section 7: Layers and Furnishing Layers - Part 2 Layers - Part 3 Furnishing Bedrooms & Bathrooms - Part 1 Adding Furniture, Fixtures, and Equipments | part 2 Adding Furniture, Fixtures, and Equipments | part 3 Drawing the Kitchen Drawing the Living Room Drawing Dining Room & Adding Casework Adding Steps and Railings Using Stretch Command Finishing Steps and patio areas Hatch command Hatch Customization Adding Plants & Finishing Floor Plan Section 8: Layout and Printing Paper Space & Page Setup Manager Working with Viewport & Using Print Command Scale & Using Multiple Viewports Locking Viewport Scale Drawing A Titleblock - Part 1 Drawing A Titleblock - Part 2 Copying the Titleblock Using Titleblock with Attributes Section 9: Roof Plan and Organization Drawing The Roof Plan Placing Roof Plan on Sheet Organizing Project Drawings & Using Layer Freeze AutoCAD Crashing And What To Do Section 10: Elevations Exterior elevations - Drawing the Outline Exterior elevations - Drawing the Openings Elevations - Detailing - Part 1 Elevations - Detailing - Part 2 Elevations - Repeating the workflow Elevations - Finishing 3rd Elevation Elevations - Drawing the final elevation Section 11: Sections Sections: Drawing Cut Lines & the Outline Sections: Placing 1st Section on Sheet Sections: Drawing Section #2 - Different Technique Sections: Adding Final Detail Section 12: Interior Elevations Interior Elevations: Drawing the Outline Interior Elevations: Placing on Sheet Interior Elevations: Using Block Clip Interior Elevations: Final Drawing Section 13: Conclusion Putting the Final Set Together Proficient AutoCAD Skills: Master essential tools for efficient drawing creation and manipulation. Accurate 2D Designs: Create precise and high-quality layouts with attention to detail. Effective Object Handling: Excel in selecting, moving, copying, and scaling objects. File Management: Organize drawings efficiently and understand different file formats. Precise Measurements: Utilize inquiry commands for accurate design analysis. Annotations and Hatching: Apply text, dimensions, and hatching to enhance drawings. Object Organization: Manage layers and object properties for organized designs. Reusable Content: Efficiently use blocks, tool palettes, and groups for productivity. Layout Optimization: Create layouts with viewports for optimal design presentation. Professional Output: Generate professional plots and PDF output for polished results. Enhanced Productivity: Explore AutoCAD toolsets and web/mobile apps for streamlined workflow. Course Overview: Learn versatile AutoCAD for Mac drawing techniques, applicable across all industries, enabling you to excel in the application. Our trainers incorporate exercises from various disciplines. Who Should Attend: Ideal for newcomers to AutoCAD for Mac, self-taught users seeking correct techniques, and those transitioning from other CAD tools. Requirements: A solid Mac OS understanding is essential, while prior CAD knowledge is not required. Pre-course preparation guidance is provided for new AutoCAD users. Highlights: Perfect for AutoCAD for Mac beginners. Gain skills for precise 2D layouts and designs. Versatile drawing techniques for all industries. Choose between in-class or live online training. Autodesk Certified Tutors: AutoCAD 1-2-1 courses. Hands-on learning with expert trainers. Lifetime post-course email support. Tailored on-request training available. Certificate: Digital certificate included. Tailored Mac Training: Excel in AutoCAD with specialized Mac training, leveraging its distinct features and interface for heightened efficiency. Comprehensive Skill Enhancement: Advance from fundamental to intermediate AutoCAD Mac proficiency, creating precise and professional 2D drawings. Industry-Relevant Expertise: Attain in-demand skills valued by employers in architecture, engineering, and design sectors. Hands-On Project Exposure: Immerse yourself in practical exercises mirroring real-world design scenarios, fostering practical competence. Lesson Recordings for Review: Access recorded lessons for reinforcement and deeper AutoCAD Mac comprehension. Ongoing Email Support: Benefit from lifetime email support, ensuring assistance beyond course completion."
Search By Location
- Print Courses in London
- Print Courses in Birmingham
- Print Courses in Glasgow
- Print Courses in Liverpool
- Print Courses in Bristol
- Print Courses in Manchester
- Print Courses in Sheffield
- Print Courses in Leeds
- Print Courses in Edinburgh
- Print Courses in Leicester
- Print Courses in Coventry
- Print Courses in Bradford
- Print Courses in Cardiff
- Print Courses in Belfast
- Print Courses in Nottingham
