- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
445 Pharmaceutical courses
Overview: ***Limited Time Flash Sale*** Get Hard Copy + PDF Certificates + Transcript + Student ID Card worth £250 as a Gift! - Enrol Now! This Phlebotomy Training bundle consists of courses that are designed to give you step-by-step tutorials on venipuncture and phlebotomy skills along with other medical training. Since the pandemic, the need for medical professionals has skyrocketed. This means there are many jobs that you can apply for. To cater to the high demand and provide an all-inclusive course for those looking to learn skills, we bring you this Phlebotomy Training bundle. As phlebotomy includes the drawing of blood, you need to be very careful and you need to know several things before doing anything. This Phlebotomy Training bundle provides you with the knowledge of venipuncture along with several other medical skills such as clinical coding, laboratory assistant training and much more. All of these skills and knowledge in the medical field will prove extremely useful for you when you apply for medical jobs in the UK. Along with this Phlebotomy Training course, you will get 19 premium courses, an original Hardcopy, 20 PDF Certificates (Main Course + Additional Courses) Student ID card as gifts. This Phlebotomy Training Bundle Consists of the following Premium courses: Course 01: Phlebotomy Diploma Course 02: Immunisation Nurse Course 03: Vaccine Management Course 04: Infection Prevention Training Course 05: Anatomy and Physiology of the Human Body Course 06: Sterile Services Technician Training Level 4 Course 07: Medical Laboratory Assistant Training Level 3 Course 08: Medication Training Course 09: Nursing Assistant Course 10: Nurse Prescribing Diploma Course 11: Medical Transcription Course 12: Medical Terminology Training Course 13: Medical Law Course 14: Level 3 Diploma in Health & Social Care Course 15: Clinical Observations Skills Course 16: Public Health Course 17: Medication Administration Course 18: First Aid Training Course 19: Personal Hygiene Course 20: Communication & Information Handling in Care Learning Outcomes of this Phlebotomy Training bundle: Master the essential techniques and procedures in Phlebotomy for safe practice. Understand the anatomy and physiology relevant to Phlebotomy procedures. Gain proficiency in handling and operating Phlebotomy equipment accurately. Learn proper patient preparation and care during Phlebotomy procedures. Develop skills to prevent and manage complications during Phlebotomy sessions. Acquire knowledge of legal and ethical standards in Phlebotomy practice. Key Features of the Phlebotomy Training Course: FREE Phlebotomy Training CPD-accredited certificate Get a free student ID card with Phlebotomy Training training (£10 applicable for international delivery) Lifetime access to the Phlebotomy Training course materials The Phlebotomy Training program comes with 24/7 tutor support Get instant access to this Phlebotomy Training Course Learn Phlebotomy Training training from anywhere in the world The Phlebotomy Training training is affordable and simple to understand The Phlebotomy Training training is an entirely online So, enroll in our Phlebotomy Training Bundle to kick-start your career! Certificates Digital certificate - Included You will get the PDF Certificate for the title course (Phlebotomy Diploma) absolutely Free! Hard copy certificate - Included You will get the Hard Copy certificate for the title course (Phlebotomy Diploma) absolutely Free! Other Hard Copy certificates are available for £14.99 each. Please Note: The delivery charge inside the UK is £3.99, and the international students must pay a £9.99 shipping cost. Description: Enroll in this Phlebotomy Training Bundle today and start your journey to become successful! ★★★ Course Curriculum of Phlebotomy Training Bundle ★★★ Course 01: Phlebotomy Diploma Module 01: Introduction Module 02: Blood Circulation, Function, and Composition Module 03: Phlebotomy Equipment Module 04: Routine Venipuncture Module 05: Venipuncture Complications and Pre-Examination Variables Module 06: Dermal Puncture Module 07: Quality Assessment and Management Module 08: Special Blood Collection Procedure Module 09: Infection Control and Risk Management =========>>>>> And 19 More Related Courses <<<<<========= How will I get my Phlebotomy Training Certificate? After successfully completing the course, you will be able to order your CPD Accredited Certificates (PDF + Hard Copy) as proof of your achievement. PDF Certificate: Free (Previously it was £9.99*20 = £199.7) Hard Copy Certificate: Free (For The Title Course: Previously it was £14.99) So, enroll now in this Phlebotomy Training Bundle to kickstart your career! Who is this course for? Anyone from any background can enroll in this Phlebotomy Training bundle. Requirements This Phlebotomy Training course has been designed to be fully compatible with tablets and smartphones. Career path This Phlebotomy Training will improve your eligibility for a number of jobs across the healthcare and medical industries such as: Phlebotomists Phlebotomy Nurses Phlebotomy Lab Technicians Phlebotomy Healthcare Workers
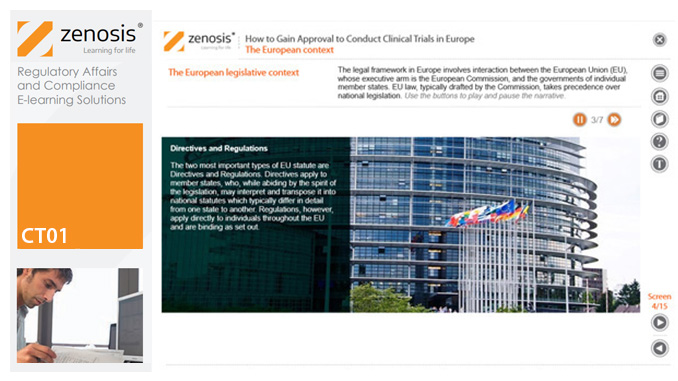
CT01: How to Gain and Maintain Approval for Clinical Research Under the EU Clinical Trials Directive
By Zenosis
To conduct a clinical trial in the European Economic Area under the Clinical Trials Directive the sponsor must apply for authorisation from the national competent authority (i.e. medicines regulator), and favourable opinion must be obtained from a research ethics committee, in each member state in which the trial is to take place. This module sets out the requirements for successful compilation, submission and maintenance of the applications.
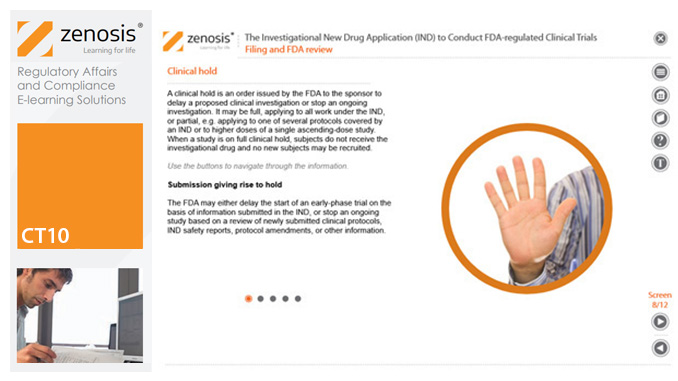
CT10: The Investigational New Drug Application (IND) to Conduct FDA-regulated Clinical Trials
By Zenosis
An Investigational New Drug Application (IND) is a submission to the US Food and Drug Administration (FDA) for permission to conduct a clinical trial of a medicinal product. This module describes regulatory requirements that sponsors or sponsor-investigators must meet for successful compilation, filing and maintenance of INDs. The IND and its role are defined, and the contexts in which it is required are specified.
CT03: ICH Good Clinical Practice
By Zenosis
Good Clinical Practice (GCP) is a set of internationally recognised ethical and scientific quality requirements for designing, conducting, recording and reporting clinical trials. Compliance with GCP principles is required by regulatory authorities in many countries for the authorisation of clinical trials and the acceptance of their data. The International Council for Harmonisation’s guideline E6, often referred to as ICH GCP, is the international standard specification for Good Clinical Practice.

Biochemical Engineering
By Compliance Central
Incredible Things Originate from a Little Bundle Special Price Cut Offer Are you looking to enhance your Biochemical Engineering skills? If yes, then you have come to the right place. Our comprehensive courses on Biochemical Engineering will assist you in producing the best possible outcome by mastering the Biochemical Engineering skills. Get 6 CPD Accredited Courses for only £41! Offer Valid for a Limited Time!! Hurry Up and Enrol Now!!! Course 01: Biomedical Science Course 02: Biochemistry Course 03: Microbiology Course 04: Biotechnology Course 05: Lab Analyst Training Course 06: Epidemiology The Biochemical Engineering bundle is for those who want to be successful. In the Biochemical Engineering bundle, you will learn the essential knowledge needed to become well versed in Biochemical Engineering. Our Biochemical Engineering bundle starts with the basics of Biochemical Engineering and gradually progresses towards advanced topics. Therefore, each lesson of this Biochemical Engineering course is intuitive and easy to understand. Why would you choose the Biochemical Engineering course from Compliance Central: Lifetime access to Biochemical Engineering courses materials Full tutor support is available from Monday to Friday with the Biochemical Engineering course Learn Biochemical Engineering skills at your own pace from the comfort of your home Gain a complete understanding of Biochemical Engineering course Accessible, informative Biochemical Engineering learning modules designed by expert instructors Get 24/7 help or advice from our email and live chat teams with the Biochemical Engineering bundle Biochemical Engineering Curriculum Breakdown of the Biochemical Engineering Bundle Curriculum Breakdown of Biomedical Science Module 01: Introduction to Biomedical Science Module 02: Genetics and Biochemistry Module 03: Microbiology and Cell Biology Module 04: Biochemical Engineering and Enzyme Discovery Module 05:Toxicology, Pharmaceuticals and Fine Chemicals Module 06: Systems and Synthetic Biology Module 07: Global Health Challenges Curriculum Breakdown of Biochemistry Module 01: Introduction to Biochemistry Module 02: Introduction to Cells Module 03: Organic Chemist And Much More... Curriculum Breakdown of Microbiology Module 01: Introduction to Microbiology Module 02: Bacteriology Module 03: Viruses And Much More... Curriculum Breakdown of Biotechnology Module 1 - Introduction to Biotechnology Module 2- Biotechnology in the Realm of History Module 3- Molecular and Cellular Biology And Much More... Curriculum Breakdown of Lab Analyst Training Module 01: Introduction to the Course Module 02: Medical Laboratory Services Module 03: Laboratory Equipments (Part-01) And Much More... Curriculum Breakdown of Epidemiology Module 01: Understanding Epidemiology Module 02: Principles of Epidemiology Module 03: Study Design And Much More... CPD 60 CPD hours / points Accredited by CPD Quality Standards Who is this course for? The Biochemical Engineering bundle helps aspiring professionals who want to obtain the knowledge and familiarise themselves with the skillsets to pursue a career in Biochemical Engineering. It is also great for professionals who are already working in Biochemical Engineering and want to get promoted at work. Requirements To enrol in this Biochemical Engineering course, all you need is a basic understanding of the English Language and an internet connection. Career path The Biochemical Engineering bundle will enhance your knowledge and improve your confidence in exploring opportunities in various sectors related to Biochemical Engineering. Biochemical Engineer: £35,000 to £70,000 per year Process Development Scientist: £30,000 to £60,000 per year Bioprocess Engineer: £35,000 to £65,000 per year Quality Control Analyst: £25,000 to £45,000 per year Certificates CPD Accredited PDF Certificate Digital certificate - Included 6 CPD Accredited PDF Certificate for Free Hard copy certificate Hard copy certificate - £9.99 CPD Accredited Hard Copy Certificate for £9.99 each. Delivery Charge: Inside the UK: Free Outside of the UK: £9.99

CT09: Good Clinical Practice Inspections and Audits
By Zenosis
The module describes general principles of GCP inspection and audit, discusses preparation for an inspection, and sets out in detail what European and US FDA inspectors will examine. Finally it describes post-inspection actions by the regulator and the inspected party.

SUB05: Electronic Common Technical Document (eCTD)
By Zenosis
The eCTD is mandatory for all applications for marketing approval and all subsequent related submissions in the European Economic Area, the USA and Canada. Other countries intend to make its use mandatory. The eCTD specification has been developed to facilitate the global electronic submission, review and lifecycle management of medicinal product dossiers for regulatory applications. It broadens the scope of the CTD to include information on variations, renewals and amendments, so that it is no longer a static document but is updatable throughout the life of the product. This module outlines the eCTD specification, discusses the approach to regional differences in dossiers, and provides guidance on creation of an eCTD submission. The module provides a training and reference tool that will be of particular value to those new to the use of the format.

Explore the world of sustainable aquaculture and fisheries management with our comprehensive course. From understanding aquatic species biology to implementing eco-friendly aquaculture systems, dive into nutrition, health management, and the economic aspects of the industry. Gain practical insights and contribute to responsible practices for a sustainable future. Join us now for an immersive learning experience in the intersection of environmental stewardship and thriving aquaculture and fisheries enterprises.

Explore the dynamic world of Industrial Microbiology: Applications and Techniques in this comprehensive course. Delve into microbial physiology, genetics, bioprocessing, and environmental applications. Gain hands-on experience with essential tools and techniques, preparing you for a future in biotechnology and industrial innovation.

Explore the intricate bond between plants and human societies in our Ethnobotany course. Uncover traditional plant uses, delve into botanical foundations, and master ethnobotanical research methods. Discover sustainability, biodiversity conservation, and future horizons in this interdisciplinary journey. Join us to deepen your understanding of the vital relationship shaping our cultural, ecological, and economic landscapes.

Search By Location
- Pharmaceutical Courses in London
- Pharmaceutical Courses in Birmingham
- Pharmaceutical Courses in Glasgow
- Pharmaceutical Courses in Liverpool
- Pharmaceutical Courses in Bristol
- Pharmaceutical Courses in Manchester
- Pharmaceutical Courses in Sheffield
- Pharmaceutical Courses in Leeds
- Pharmaceutical Courses in Edinburgh
- Pharmaceutical Courses in Leicester
- Pharmaceutical Courses in Coventry
- Pharmaceutical Courses in Bradford
- Pharmaceutical Courses in Cardiff
- Pharmaceutical Courses in Belfast
- Pharmaceutical Courses in Nottingham