- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
445 Pharmaceutical courses
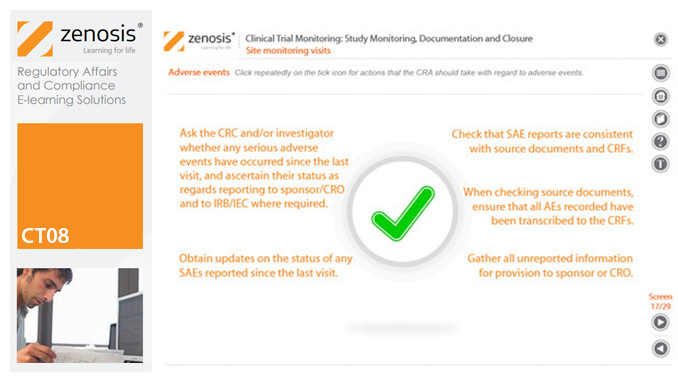
CT08: Clinical Trial Monitoring: Study Monitoring, Documentation and Closure
By Zenosis
The sponsor of a clinical trial must arrange for it to be monitored throughout its duration to ensure that the rights and wellbeing of subjects are protected, the trial data are accurate, complete and verified from source documents, and the conduct of the trial complies with the study protocol, Good Clinical Practice and regulatory requirements. In this module we describe how a Clinical Research Associate (CRA) monitors an ongoing trial to its conclusion.
PKPD02: Conducting Pharmacokinetic and Pharmacodynamic Studies
By Zenosis
This module extends the learner’s understanding of pharmacokinetic and pharmacodynamic studies from the basics described in our companion module PKPD01, An Introduction to Pharmacokinetics and Pharmacodynamics in Drug Development and Registration. It provides detail on a variety of aspects of such studies: design, sampling, data analysis, research in special populations, and bioequivalence testing.

SAM02: Regulatory Requirements and Guidance on Advertising and Promotion of Prescription Drugs in the USA
By Zenosis
In this course we explain how to advertise and promote prescription drugs in various media, whether to healthcare professionals or consumers, in compliance with legal requirements and guidance from the FDA.

SAM04: Marketing of Prescription Drugs in the USA – Interactions with Healthcare Professionals
By Zenosis
The heaviest legal penalties imposed on drug companies concern interactions with healthcare professionals in the context of prescription drug marketing, notably for violations of the Anti-Kickback Statute and the False Claims Act. Monetary penalties have amounted to billions of dollars in some cases.

CT11: How to Gain Authorisation for Clinical Research Under the EU Clinical Trials Regulation
By Zenosis
This course sets out the procedures that sponsors need to follow to gain authorisation to conduct clinical trials under the Regulation, and it summarises and links to the extensive guidance available from the European Commission and the European Medicines Agency. Its companion course CT12 sets out the procedures that sponsors need to follow to conduct authorised clinical trials in compliance with the Regulation. The two courses therefore provide an ideal foundation for understanding and complying with the new law.
CT13: Safety Reporting in Clinical Trials
By Zenosis
This course explains the regulatory requirements for the reporting of adverse events and suspected adverse reactions in clinical trials. It describes how investigators should report to sponsors, and how sponsors should report to regulatory authorities and other stakeholders in the safety of investigational products. It explains how events are characterized as serious or non-serious, expected or unexpected, and it distinguishes the requirements for each category. It describes controlled vocabularies used for coding of events in reports.
CT14: Clinical Trial Safety Reporting Requirements in the EU and USA
By Zenosis
This course sets out the legal and regulatory requirements for safety reporting in clinical trials of medicinal products under the jurisdictions of the European Union and the USA. It builds on the foundation laid by our companion course CT13, Safety Reporting in Clinical Trials, and provides greater detail of specific requirements in those jurisdictions.

SAM03: Consumer-directed Advertising and Online Promotion of Prescription Drugs in the USA
By Zenosis
Unlike the great majority of other countries, the advertising of prescription drugs directly to consumers is permitted in the USA. Spending by drug companies on direct-to-consumer (DTC) advertising of prescription drugs has increased more than four-fold over two decades, with a dramatic increase in the number of TV ads. In addition, the Internet and social media platforms have increasingly enabled companies to engage more actively with the public.

SAM01: Legal and Regulatory Framework for Advertising and Promotion of Prescription Drugs in the USA
By Zenosis
In this course we set out the legal framework for the regulation of advertising and promotion of prescription drugs in the USA. We identify the regulatory authorities and sources of guidance. We summarize basic requirements that advertisements and promotional labeling must meet, and we identify consequences that may follow failure to comply.

Introduction to Cannabis-Based Products for Medicinal Use (CBPM), prescription and regulations
By Medisonal Institute of Education
Discover the history and basic pharmacology of medical cannabis and learn about the full prescription process and current regulations surrounding CBPM in the UK. To recognise the origins of cannabis use and to recall key properties leading to its historical use. To demonstrate the process of prescribing medical cannabis and list what is required pre-, during-, and post- prescription. To summarise the regulatory landscape surrounding CBPM and successfully interpret the law regarding medical use.

Search By Location
- Pharmaceutical Courses in London
- Pharmaceutical Courses in Birmingham
- Pharmaceutical Courses in Glasgow
- Pharmaceutical Courses in Liverpool
- Pharmaceutical Courses in Bristol
- Pharmaceutical Courses in Manchester
- Pharmaceutical Courses in Sheffield
- Pharmaceutical Courses in Leeds
- Pharmaceutical Courses in Edinburgh
- Pharmaceutical Courses in Leicester
- Pharmaceutical Courses in Coventry
- Pharmaceutical Courses in Bradford
- Pharmaceutical Courses in Cardiff
- Pharmaceutical Courses in Belfast
- Pharmaceutical Courses in Nottingham