- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
445 Pharmaceutical courses
***Don't Spend More; Spend Smart*** Life Just Got Better This Summer! Get Hard Copy + PDF Certificates + Transcript + Student ID Card + e-Learning App as a Gift - Enrol Now Tired of browsing and searching for the course you are looking for? Can't find the complete package that fulfils all your needs? Then don't worry as you have just found the solution. Take a minute and look through this 14-in-1 extensive bundle that has everything you need to succeed in Materials Science and other relevant fields! After surveying thousands of learners just like you and considering their valuable feedback, this all-in-one Materials Science bundle has been designed by industry experts. We prioritised what learners were looking for in a complete package and developed this in-demand Materials Science course that will enhance your skills and prepare you for the competitive job market. Also, our Materials Science experts are available for answering your queries and help you along your learning journey. Advanced audio-visual learning modules of these courses are broken down into little chunks so that you can learn at your own pace without being overwhelmed by too much material at once. Furthermore, to help you showcase your expertise in Materials Science, we have prepared a special gift of 1 hardcopy certificate and 1 PDF certificate for the title course completely free of cost. These certificates will enhance your credibility and encourage possible employers to pick you over the rest. This Materials Science Bundle Consists of the following Premium courses: Course 01: Basic Chemistry Online Course Course 02: Organic Chemistry Course 03: Organic Chemistry Demystified Course 04: General Inorganic Chemistry Course 05: Particle Physics Course 06: Biochemistry Course 07: Biotechnology Course 08: Biomedical Science Course 09: Diploma in Water Chemistry Course 10: Lab Analyst and Laboratory Skills Level 5 Course 11: COSHH Training and Safety - Level 2 Course 12: Gas Safety Course 13: Fire Safety | Online Course Course 14: Health, Safety & Laws at Workplace | Online Course How will I get my Certificate? After successfully completing the course, you will be able to order your CPD Accredited Certificates (PDF + Hard Copy) as proof of your achievement. PDF Certificate: Free (For The Title Course) Hard Copy Certificate: Free (For The Title Course) Curriculum of Materials Science Bundle Course 01: Basic Chemistry Online Course Introduction to Basic Chemistry Understanding Matter Atom Chemical Bonding Energy Periodic Table Intermolecular Forces Gases Solution Chemical Equilibrium Kinetics and Equilibrium Acids and Bases pH and pOH Reactions Involving Acids and Bases Redox Reaction Electricity and Chemistry Inorganic Chemistry Organic Chemistry Biochemistry Nuclear Chemistry Industrial Chemistry Environmental Chemistry Laboratory Safety Chemical Hygiene Plan Course 02: Organic Chemistry Alkane series Alkene Series Alkanol Series Alkyne series Alkanals and Aldehydes Nomenclature Practices Course 03: Organic Chemistry Demystified About the Course Drawing Organic Molecules Resonance Acid-Base Reactions Geometry Nomenclature Conformations Configurations Mechanisms Substitution Reactions Elimination Reactions Substitution vs Elimination Addition Reactions Synthesis Techniques Wrapping Up and Bonus Course 04: General Inorganic Chemistry ABOUT THE COURSE Quantities and Units Atomic, Molecular and Molar Masses Formulas and Compositions Inorganic Nomenclature Chemical Equations Measurement of Gases The Ideal Gas Law Thermochemistry Atomic Structure Chemical Bonding and Molecular Structure Solids and Liquids Oxidation - Reduction Concentration of Solutions Reaction Involving Standard Solutions Properties of Solutions Thermodynamics Acids and Bases Complex Ions and Precipitates Electrochemistry Rates of Reactions Course 05: Particle Physics Introduction to Particle Physics Elementary Particles The Nucleus Nuclear Physics Particle Accelerators Radiation detectors The Standard Model Course 06: Biochemistry Introduction to Biochemistry Introduction to Cells Organic Chemistry Biomolecules Protein Biomolecules of Nucleic Acids Genetics Metabolism Course 07: Biotechnology Introduction to Biotechnology Biotechnology in the Realm of History Molecular and Cellular Biology Biotechnology Versus Molecular Biology Genetic Engineering Biotechnology in Health Sector Industrial Biotechnology Biotechnology in a Business Perspective Course 08: Biomedical Science Introduction to Biomedical Science Genetics and Biochemistry Microbiology and Cell Biology Biochemical Engineering and Enzyme Discovery Toxicology, Pharmaceuticals and Fine Chemicals Systems and Synthetic Biology Global Health Challenges Course 09: Diploma in Water Chemistry Introduction to Water Chemistry Concepts of Basic Chemistry Alkalinity of Water Hard Water and Basic Water Treatment Principles Water Treatment and Purification Chemical Processes of Water Treatment Inorganic Chemicals Volatile and Synthetic Organic Compounds Metalloid Section Metals and Heavy Metal Section Laboratory Safety Chemical Hygiene Plan Course 10: Lab Analyst and Laboratory Skills Level 5 Introduction to the Course Medical Laboratory Services Laboratory Equipments (Part-01) Laboratory Equipments (Part-02) Microscope and How to Use It Solutions Used in Clinical/Medical Laboratory Sterilisation, Disinfection and Decontamination Basic Haematology Basic Haemostasis (Coagulation) Basic Immunology and Immunohematology Urinalysis Basic Clinical Chemistry Basic Clinical Microbiology Basic Parasitology Laboratory Accidents and Safety Quality Assurance Course 11: COSHH Training and Safety - Level 2 COSHH Explained COSHH Regulations Health Problems Related to Hazardous Substances Identifying Hazardous Substances COSHH Risk Assessments Control Measures and Monitoring Implementing Control Measures Course 12: Gas Safety Gas Safety Legislation Combustion Transmission System: Pipes and Flues Ventilation Tightness Testing Meter Regulator Check Appliance Pressures and Gas Rates Emergency Isolation Controls and Valves Gas Safety Devices and Controls Gas Warning Labelling Emergency and Evacuation Procedures Chimney Standards, Inspection and Testing Gas Appliances and Safety Course 13: Fire Safety | Online Course Basics of Fire Safety Regulations and Risk Assessment Fire Safety Measures Fire Safety routes and signs Evacuation Strategies Course 14: Health, Safety & Laws at Workplace | Online Course Workplace Health and Safety Laws Managing for Health and Safety Risk Assessment and Common Risks Workplace Health, Safety and Welfare Accidents and Ill Health at Work Electrical, Fire and Gas Safety Safety in Different Work Settings Work Equipment Hazards and Risk Control Other Health and Safety Hazards at Work CPD 150 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Anyone from any background can enrol in this Materials Science bundle. Persons with similar professions can also refresh or strengthen their skills by enrolling in this Materials science course. Students can take this course to gather professional knowledge besides their study or for the future. Requirements Our Materials Science is fully compatible with PC's, Mac's, laptops, tablets and Smartphone devices. This Materials science course has been designed to be fully compatible with tablets and smartphones so that you can access your course on Wi-Fi, 3G or 4G. There is no time limit for completing this Materials science course; it can be studied in your own time at your own pace. Career path Having this Materials science expertise will increase the value of your CV and open you up to multiple job sectors. Certificates Certificate of completion Digital certificate - Included
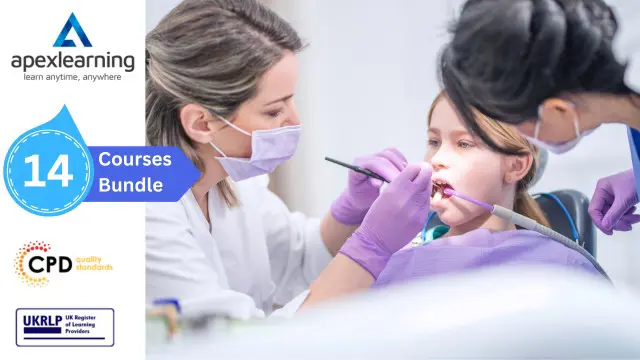
Dental Hygienist | Radiography | Human Anatomy and Physiology | Wound Care | Medical Transcription | Medication Administration | Medical Law | Sterile Services Technician | Infection Prevention | Diabetes Care and more... If you've put a smile on someone's face today, you've done more good than you know. Dental hygienists are the people who help us keep our smiles. In addition, they educate patients about oral health. However, dental hygienists are mainly concerned with 'preventive' dental health and treating gum disease - showing us correct home care and helping us to keep our teeth and gums healthy. This includes professionally cleaning our teeth by removing plaque and tartar (usually called a 'scale and polish' or prophylaxis). Dental hygienists are in demand in the United Kingdom as schools struggle to meet the population's needs. In addition, dental hygiene provides a respectable income in England. Starting salaries for dental hygienists working in the NHS are typically between £24,907 and £30,615. This Dental Hygienist bundle will help new dentists kickstart their careers via essential knowledge, skillsets and confidence. Learn the fundamentals of Dental Hygienists to carry out different kinds of oral-care procedures. Have the ability to operate medical imaging technology for diagnostic and treatment purposes, including dental X-rays (radiographs). In addition, understand the responsibilities of a medical administrator and gain invaluable skills like organising schedules, maintaining medical records, and the legal aspects and ethics. Furthermore, to help you showcase your expertise in Dental Hygienist, we have prepared a special gift of 1 hardcopy certificate and 1 PDF certificate for the title course completely free of cost. These certificates will enhance your credibility and encourage possible employers to pick you over the rest. This Dental Hygienist Bundle Consists of the following Premium courses: Course 01: Dental Hygienist Course 02: Radiography Course 03: Sterile Services Technician Training Level 4 Course 04: Medical Terminology Training Course 05: Medical Transcription Course 06: Medication Administration Level 4 Course 07: GDPR in Healthcare Course 08: European Medical Device Regulations Course 09: Medical Law Course 10: Diabetes Care Diploma Course 11: Immunisation Nurse Course 12: Infection Prevention Training Course 13: Personal Hygiene Course 14: First Aid Trainer Course Benefits you'll get choosing Apex Learning for this Dental Hygienist: Pay once and get lifetime access to 14 CPD courses Free e-Learning App for engaging reading materials & helpful assistance Certificates, student ID for the title course are included in a one-time fee Free up your time - don't waste time and money travelling for classes Accessible, informative modules designed by expert instructors Learn at your ease - anytime, from anywhere Study the course from your computer, tablet or mobile device CPD accredited course - improve the chance of gaining professional skills How will I get my Certificate? After successfully completing the course you will be able to order your CPD Accredited Certificates (PDF + Hard Copy) as proof of your achievement. PDF Certificate: Free (For The Title Course) Hard Copy Certificate: Free (For The Title Course) Course 01: Dental Hygienist General Dentistry Instruments Dental Office Management Oral Diagnosis, Treatment Planning and Dental Terminology General Handling and Safety of Dental Materials in the Dental Office Infection Control and Instruments Paediatric Dentistry Standards of Dental Practice in the UK Course 02: Radiography An Introduction to Radiographic Science Radiological Physics Radiobiology Radiation Protection The Digital Radiographic Image Dental Radiography Radiography of Different Body Parts Course 03: Sterile Services Technician Training Level 4 Introduction Universal Precautions The Laminar Air Flow Hood (LAF) 3 lectures Sterile Compounding Pharmaceuticals Demonstrations Compounding Pharmacy Math BONUS REVIEW OF THE STERILE COMPOUNDING PROCESS Course 04: Medical Terminology Training Human Body Vocabulary with Pictures Equipment, Instruments and Lab Tests Acronyms and Abbreviation Digestive System Cardiovascular System Blood and Immunity System Respiratory System Musculoskeletal System Central Nervous System Course 05: Medical Transcription Introduction to Medical Transcription The What, How, Who and Why of Medical Transcription Understanding Medical Reports Medical Language Boot Camp Formatting: The Basics Data Protection Course 06: Medication Administration Level 4 Introduction to Safe Handling of Medicines Legislation and guidance of medication management Principles of Safe and Appropriate Handling of Medicines Medication Risk Assessment Guidance Handling Medicines in Social Care Settings Consent to Treatment Requirements for Specific Services Levels of Care and Support Procedure for Handling Medication The Six Rights of Medication Administration Covert Administration of Medication Recording Procedures Storage of Medication Transfer and Disposal of Medication Drug Formulation and Ways of Taking It Course 07: GDPR in Healthcare Introduction to GDPR GDPR and Healthcare Setting General Data Protection Regulations Explained Lawful Basis for Preparation Responsibilities and Obligations Electronic Medical Records Rights and Breaches Course 08: European Medical Device Regulations Overview to European Regulations on Medical Devices Essential Components of EU MDR Reporting Requirements and Identification Quality System in Medical Device Regulation Course 09: Medical Law An Introduction to Medical Law Legislation on Access to Health, Medical Report, Treatment Legislation on Adult Support Legislation on Public Health and Health Service (Part 1) Legislation on Public Health and Health Service (Part 2) Legislation on Public Health and Health Service (Part 3) Legislation on Public Health and Health Service (Part 4) Legislation on Coronavirus Legislation on Mental Health (Part 1) Legislation on Mental Health (Part 2) Legislation on Abortion Other Legislation (Part 1) Other Legislation (Part 2) Course 10: Diabetes Care Diploma What is Diabetes The Diabetes Challenge The Cost of Diabetes in Social Care Type 1 Diabetes Type 2 Diabetes Type 2 Diabetes Treatments Prediabetes Gestational Diabetes Other Types of Diabetes Hypoglycaemia Hyperglycaemia and Hyperosmolar Hyperglycaemic State Glossary of Diabetes Terms Course 11: Immunisation Nurse Introduction to Nursing Immunology and Immunisation Understanding Immune System in Nursing Vaccine and Vaccination Types of Vaccine Vaccine Safety Working in Different Healthcare Settings Effective Communication in Nursing Health and Safety in Nursing Hygiene in Nursing Infection Control Course 12: Infection Prevention Training Infection Prevention and Control Policy in the UK Principles of Prevention and Control of Infection Immunization Infections Spread by Food and Water Infections Spread by Animals and Insects, and Less Common Infections Found in the UK Infections & Diseases Spread by Person-to-Person Contact Infections Spread by Sexual Contact Infections Spread by Blood and Body Fluids Course 13: Personal Hygiene Introduction to Personal Hygiene and a Brief History Looking Clean, Smelling Clean Improving and Maintaining Oral Health Care of the Eyes Ear Care Secrets Foot & Nail Care in Personal Hygiene Hair Care Hygienic Ways Hygienic Approach to Skin Care Maintain Good Toilet Hygiene Potential Hygiene Guidelines Personal Hygiene During Coronavirus Pandemic Course 14: First Aid Trainer Course Introduction to First Aid Training Classifications and Abbreviations Primary Survey Secondary Survey Incidents Management Basic First-Aid Techniques Dealing with Minor Injuries Dealing with Major Injuries Dealing With Fractures and Dislocations Call for an Emergency Sports First Aid First Aid at Work Place And Much More... CPD 145 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Anyone from any background can enrol in this bundle. Persons with similar professions can also refresh or strengthen their skills by enrolling in this course. Students can take this course to gather professional knowledge besides their study or for the future. Requirements Our is fully compatible with PC's, Mac's, laptops, tablets and Smartphone devices. This course has been designed to be fully compatible with tablets and smartphones so that you can access your course on Wi-Fi, 3G or 4G. There is no time limit for completing this course; it can be studied in your own time at your own pace. Career path Having this various expertise will increase the value of your CV and open you up to multiple job sectors. Certificates Certificate of completion Digital certificate - Included You will get the PDF Certificate for the title course (Dental Hygienist) absolutely Free! Certificate of completion Hard copy certificate - Included You will get the Hard Copy certificate for the title course (Dental Hygienist) absolutely Free! Other Hard Copy certificates are available for £10 each. Please Note: The delivery charge inside the UK is £3.99, and the international students must pay a £9.99 shipping cost.
Give a compliment to your career and take it to the next level. This Human Biology bundle will provide you with the essential knowledge to shine in your professional career. Whether you want to develop skills for your next job or elevate your skills for your next promotion, this Human Biology bundle will help you stay ahead of the pack. Throughout the Human Biology programme, it stresses how to improve your competency as a person in your chosen field while also outlining essential career insights in the relevant job sector. Along with this Human Biology course, you will get 10 premium courses, an originalhardcopy, 11 PDF certificates (Main Course + Additional Courses) Student ID card as gifts. This Human Biology Bundle Consists of the following Premium courses: Course 01: Basic Biology Course Course 02: Biomedical Science Course 03: Biotechnology Course 04: Genetics Course 05: Diploma In Microbiology Course 06: Microbiology Technician Course Course 07: Human Anatomy and Physiology Course 08: Lab Analyst Training Course 09: Biochemistry Course 10: Sterile Services Technician Training Level 4 Course 11: Medical Terminology Training Enrol now in Human Biology to advance your career, and use the premium study materials from Apex Learning. How will I get my Certificate? After successfully completing the Human biology course, you will be able to order your CPD Accredited Certificates (PDF + Hard Copy) as proof of your achievement. PDF Certificate: Free (Previously it was £10 * 11 = £110) Hard Copy Certificate: Free (For The Title Course) PS The delivery charge inside the UK is £3.99, and the international students have to pay £9.99. The bundle incorporates basic to advanced level skills to shed some light on your way and boost your career. Hence, you can strengthen your Human Biology expertise and essential knowledge, which will assist you in reaching your goal. Moreover, you can learn from any place in your own time without travelling for classes. Curriculum of Bundle Course 01: Basic Biology Course Module 01: Introduction to Biology Module 02: Characteristics, Classification and Organisation of Living Organisms Module 03: The Biosphere and Humans Module 04: Evolution of Life Module 05: The Ecology Module 06: Cells to Organs and Function of Organs Module 07: Human Body Module 08: The Plants Course 02: Biomedical Science Module 01: Introduction to Biomedical Science Module 02: Genetics and Biochemistry Module 03: Microbiology and Cell Biology Module 04: Biochemical Engineering and Enzyme Discovery Module 05:Toxicology, Pharmaceuticals and Fine Chemicals Module 06: Systems and Synthetic Biology Module 07: Global Health Challenges Course 03: Biotechnology Module 1 - Introduction to Biotechnology Module 2- Biotechnology in the Realm of History Module 3- Molecular and Cellular Biology Module 4 - Biotechnology Versus Molecular Biology Module 5- Genetic Engineering Module 6- Biotechnology in Health Sector Module 7- Industrial Biotechnology Module 8- Biotechnology in a Business Perspective Course 04: Genetics Module 01- Fundamental Concepts of Genetics Module 02- Genetics and Genomics Module 03- Genetic Disorders Module 04- Genetic Counselling Module 05- Application of Genetics Knowledge Course 05: Diploma In Microbiology Module 01 : Introduction to Microbiology Module 02 : Cell Structure and Organisation Module 03 : The Chemical Metabolism Module 04 : Microbial Growth Controlling Module 05 : Microbial Diversity Prokaryote Diversity Module 06 : Microbial Diversity Eukaryote Diversity Module 07 : Viruses, Viroids, and Prions Module 08 : Interactions between Microbes and Humans Module 09 : Human Microbial Diseases Module 10 : Immunity Module 11 : Vaccines and Antimicrobial Agents Module 12 : Microorganisms in the Environment Module 13 : Industrial and Food Microbiology Course 06: Microbiology Technician Course Module 01 : Introduction to Microbiology Module 02 : Cell Structure and Organisation Module 03 : Microbial Diversity Prokaryote Diversity Module 04 : Microbial Diversity Eukaryote Diversity Module 05 : Viruses, Viroids, and Prions Module 06 : Interactions between Microbes and Humans Module 07 : Human Microbial Diseases Module 08 : Microorganisms in the Environment Module 09: Laboratory Equipments (Part-01) Module 10: Laboratory Equipments (Part-02) Module 11: Solutions Used in Clinical/Medical Laboratory Module 12: Sterilisation, Disinfection and Decontamination Module 13: Laboratory Accidents and Safety Course 07: Human Anatomy and Physiology Module 01: Human Body Vocabulary with Pictures Module 02: Equipment, Instruments and Lab Tests Module 03: Acronyms and Abbreviation Module 04: Digestive System Module 05: Cardiovascular System Module 06: Blood and Immunity System Module 07: Respiratory System Module 08: Musculoskeletal System Module 09: Central Nervous System Course 08: Lab Analyst Training Introduction to the Course Medical Laboratory Services Laboratory Equipments (Part-01) Laboratory Equipments (Part-02) Microscope and How to Use It Solutions Used in Clinical/Medical Laboratory Sterilisation, Disinfection and Decontamination Basic Haematology Basic Haemostasis (Coagulation) Basic Immunology and Immunohematology Urinalysis Basic Clinical Chemistry Basic Clinical Microbiology Basic Parasitology Laboratory Accidents and Safety Quality Assurance Course 09: Biochemistry Module 01: Introduction to Biochemistry Module 02: Introduction to Cells Module 03: Organic Chemistry Module 04: Biomolecules Module 05: Protein Module 06: Biomolecules of Nucleic Acids Module 07: Genetics Module 08: Metabolism Course 10: Sterile Services Technician Training Level 4 Introduction Universal Precautions The Laminar Air Flow Hood (LAF) 3 lectures Sterile Compounding Pharmaceuticals Demonstrations Compounding Pharmacy Math BONUS REVIEW OF THE STERILE COMPOUNDING PROCESS Course 11: Medical Terminology Training Module 1 : Human Body Vocabulary with Pictures Module 2 : Equipment, Instruments and Lab Tests Module 3 : Acronyms and Abbreviation Module 4 : Digestive System Module 5 : Cardiovascular System Module 6 : Blood and Immunity System Module 7 : Respiratory System Module 8 : Musculoskeletal System Module 9 : Central Nervous System CPD 110 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Anyone from any background can enrol in this Human Biology bundle. Requirements This Human Biology course has been designed to be fully compatible with tablets and smartphones. Career path Having this expertise will increase the value of your CV and open you up to multiple job sectors. Certificates Certificate of completion Digital certificate - Included Certificate of completion Hard copy certificate - Included P.S. The delivery charge inside the UK is £3.99, and the international students have to pay £9.99.

Boost Your Career with Apex Learning and Get Noticed By Recruiters in this Hiring Season! Get Hard Copy + PDF Certificates + Transcript + Student ID Card worth £160 as a Gift - Enrol Now Give a compliment to your career and take it to the next level. This Dentistry will provide you with the essential knowledge and skills required to shine in your professional career. Whether you want to develop skills for your next job or want to elevate skills for your next promotion, this Dentistry will help you keep ahead of the pack. The Dentistry incorporates basic to advanced level skills to shed some light on your way and boost your career. Hence, you can reinforce your professional skills and knowledge, reaching out to the level of expertise required for your position. Further, this Dentistry will add extra value to your resume to stand out to potential employers. Throughout the programme, it stresses how to improve your competency as a person in your profession while at the same time it outlines essential career insights in this job sector. Consequently, you'll strengthen your knowledge and skills; on the other hand, see a clearer picture of your career growth in future. By the end of the Dentistry, you can equip yourself with the essentials to keep you afloat into the competition. Along with this Dentistry course, you will get 10 other premium courses. Also, you will get an original Hardcopy and PDF certificate for the title course and a student ID card absolutely free. This Dentistry Bundle Consists of the following Premium courses: Course 01: Dental Assistant Course 02: Medical Receptionist: Responsibilities and Skills Course 03: Sterile Services Technician Training Level 4 Course 04: Medical Law Course 05: Wound Care Level 2 Course 06: Medical Transcription Course 07: Medication Administration Level 4 Course 08: GDPR in Healthcare Course 09: Workplace Mental Health First Aid and Advocacy Training Course 10: Immunisation Nurse Course 11: Infection Prevention Training As one of the top course providers in the UK, we're committed to providing you with the best educational experience possible. Our industry experts have designed the Dentistry to empower you to learn all at once with accuracy. You can take the course at your own pace - anytime, from anywhere. So, enrol now to advance your career! Benefits you'll get choosing Apex Learning for this Dentistry: One payment, but lifetime access to 11 CPD courses Certificate, student ID for the title course included in a one-time fee Full tutor support available from Monday to Friday Free up your time - don't waste time and money travelling for classes Accessible, informative modules taught by expert instructors Learn at your ease - anytime, from anywhere Study the course from your computer, tablet or mobile device CPD accredited course - improve the chance of gaining professional skills Gain valuable experience without leaving your home How will I get my Certificate? After successfully completing the course you will be able to order your CPD Accredited Certificates (PDF + Hard Copy) as proof of your achievement. PDF Certificate: Free (Previously it was £10 * 11 = £110) Hard Copy Certificate: Free (For The Title Course) If you want to get hardcopy certificates for other courses, generally you have to pay £20 for each. But this Fall, Apex Learning is offering a Flat 50% discount on hard copy certificates, and you can get each for just £10! P.S. The delivery inside the U.K. is Free. International students have to pay a £3.99 postal charge. Curriculum of Dentistry Bundle Course 01: Dental Assistant Introduction to Dental Assisting General Dentistry Instruments Dental Office Management Oral Diagnosis, Treatment Planning and Dental Terminology General Handling and Safety of Dental Materials in the Dental Office Infection Control and Instruments Dental Radiology Paediatric Dentistry The Professional Dental Assistant Standards of Dental Practice in the UK Course 02: Medical Receptionist: Responsibilities and Skills Introduction to Healthcare and Receptionists Organise Schedules Maintaining Medical Records Effective Filing Systems in Medical Offices Confidentiality in a Medical Environment Time Management Medical Jargon and Terminology Maintaining Workplace Safety Information Technology in Health Care Legal aspects and Ethics Course 03: Sterile Services Technician Training Level 4 Introduction Universal Precautions The Laminar Air Flow Hood (LAF) 3 lectures Sterile Compounding Pharmaceuticals Demonstrations Compounding Pharmacy Math BONUS REVIEW OF THE STERILE COMPOUNDING PROCESS Course 04: Medical Law An Introduction to Medical Law Legislation on Access to Health, Medical Report, Treatment Legislation on Adult Support Legislation on Public Health and Health Service (Part 1) Legislation on Public Health and Health Service (Part 2) Legislation on Public Health and Health Service (Part 3) Legislation on Public Health and Health Service (Part 4) Legislation on Coronavirus Legislation on Mental Health (Part 1) Legislation on Mental Health (Part 2) Legislation on Abortion Other Legislation (Part 1) Other Legislation (Part 2) Course 05: Wound Care Level 2 The Wound Healing Process & Factors Delaying Wound Healing Types of Wounds and Their Treatment Dressing Wound Assessment Glossary Course 06: Medical Transcription Introduction to Medical Transcription The What, How, Who and Why of Medical Transcription Understanding Medical Reports Medical Language Boot Camp Formatting: The Basics Data Protection Course 07: Medication Administration Level 4 Introduction to Safe Handling of Medicines Legislation and guidance of medication management Principles of Safe and Appropriate Handling of Medicines Medication Risk Assessment Guidance Handling Medicines in Social Care Settings Consent to Treatment Requirements for Specific Services Levels of Care and Support Procedure for Handling Medication The Six Rights of Medication Administration Covert Administration of Medication Recording Procedures Storage of Medication Transfer and Disposal of Medication Drug Formulation and Ways of Taking It Course 08: GDPR in Healthcare Introduction to GDPR GDPR and Healthcare Setting General Data Protection Regulations Explained Lawful Basis for Preparation Responsibilities and Obligations Electronic Medical Records Rights and Breaches Course 09: Workplace Mental Health First Aid and Advocacy Training Introduction to Mental Health Overview of Mental Health First Aid Childhood and Adolescent Psychiatric Disorders Stress and Anxiety Disorders Schizophrenia Personality Disorders Mood Disorders Eating & Sleeping Disorders Self-Harm and Suicide Medication and Therapy in the Treatment of Mental Illness Social Attitudes to Mental Illness Mental Health Legislation and Services Mental Health in the Workplace Approach and React To People in Mental Health Crisis Course 10: Immunisation Nurse Introduction to Nursing Immunology and Immunisation Understanding Immune System in Nursing Vaccine and Vaccination Types of Vaccine Vaccine Safety Working in Different Healthcare Settings Effective Communication in Nursing Health and Safety in Nursing Hygiene in Nursing Infection Control Course 11: Infection Prevention Training Infection Prevention and Control Policy in the UK Principles of Prevention and Control of Infection Immunization Infections Spread by Food and Water Infections Spread by Animals and Insects, and Less Common Infections Found in the UK Infections & Diseases Spread by Person-to-Person Contact Infections Spread by Sexual Contact Infections Spread by Blood and Body Fluids CPD 110 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Anyone from any background can enrol in this Dentistry bundle. Persons with similar professions can also refresh or strengthen their skills by enrolling in this course. Students can take this course to gather professional knowledge besides their study or for the future. Requirements Our Dentistry is fully compatible with PC's, Mac's, Laptop, Tablet and Smartphone devices. This course has been designed to be fully compatible with tablets and smartphones so you can access your course on Wi-Fi, 3G or 4G. There is no time limit for completing this course, it can be studied in your own time at your own pace. Career path Having these various expertise will increase the value in your CV and open you up to multiple job sectors. Certificates Certificate of completion Digital certificate - Included

Give a compliment to your career and take it to the next level. This Epidemiology: Understanding the Spread and Control of Diseases will provide you with the essential knowledge and skills required to shine in your professional career. Whether you want to develop skills for your next job or want to elevate skills for your next promotion, this Epidemiology: Understanding the Spread and Control of Diseases will help you keep ahead of the pack. The Epidemiology: Understanding the Spread and Control of Diseases incorporates basic to advanced level skills to shed some light on your way and boost your career. Hence, you can reinforce your professional skills and practical knowledge, reaching out to the level of expertise required for your position. Further, this Epidemiology: Understanding the Spread and Control of Diseases will add extra value to your resume to stand out to potential employers. Throughout the programme, it stresses how to improve your competency as a person in your profession while at the same time it outlines essential career insights in this job sector. Consequently, you'll strengthen your knowledge and skills; on the other hand, see a clearer picture of your career growth in future. By the end of the Epidemiology: Understanding the Spread and Control of Diseases, you can equip yourself with the essentials to keep you afloat into the competition. Along with this Epidemiology: Understanding the Spread and Control of Diseases course, you will get 10 other premium courses. Also, you will get an original Hardcopy and PDF certificate for the title course and a student ID card absolutely free. This Epidemiology: Understanding the Spread and Control of Diseases Bundle Consists of the following Premium courses: Course 01: Epidemiology Course 02: Public Health Course 03: Immunisation Nurse Course 04: COVID-19 Critical Care Course 05: Post COVID Mental Aid Course Course 06: Medical Laboratory Assistant Training Level 3 Course 07: Biochemistry Course 08: Sterile Services Technician Training Level 4 Course 09: Infection Prevention Training Course 10: Coronavirus (COVID-19) Awareness Course 11: Personal Hygiene So, enrol now to advance your career! Benefits you'll get choosing Apex Learning: One payment, but lifetime access to 11 CPD courses Certificate, student ID for the title course included in a one-time fee Full tutor support available from Monday to Friday Free up your time - don't waste time and money travelling for classes Accessible, informative modules taught by expert instructors Get 24/7 help or advice from our email and live chat teams Learn at your ease - anytime, from anywhere Study the course from your computer, tablet or mobile device CPD accredited course - improve the chance of gaining professional skills Gain valuable and internationally renowned experience without leaving your home How will I get my Certificate? After completing the course you will be able to order your CPD Accredited Certificates (PDF + Hard Copy) as proof of your achievement. PDF Certificate: Free (Previously it was £10 * 11 = £110) Hard Copy Certificate: Free (For The Title Course) If you want to get hardcopy certificates for other courses, generally you have to pay £20 for each. But this Fall, Apex Learning is offering a Flat 50% discount on hard copy certificates, and you can get each for just £10! P.S. The delivery inside the U.K. is Free. International students have to pay a £3.99 postal charge. Curriculum of Bundle Course 01: Epidemiology Module 01: Understanding Epidemiology Module 02: Principles of Epidemiology Module 03: Study Design Module 04: Disease Occurrence Module 05: Variation in Disease by Time, Place, and Person Module 06: Epidemics and Outbreaks Module 07: Measures of Risk and Disease Prevention Module 08: Measures of Disease Frequency Module 09: Role of Epidemiology in Public Health Module 10: The Epidemiological Concept of Population Course 02: Public Health Module 01: Introduction to Public Health Module 02: Principles of Public Health Module 03: Understanding Epidemiology Module 04: Disease Control Module 05: Understanding Measures of Disease Frequency Module 06: Maternity and Childbirth Module 07: Environment and Public Health Module 08: Health System and Policy Module 09: Public Health and Ethics Course 03: Immunisation Nurse Module 01: Introduction to Nursing Module 02: Immunology and Immunisation Module 03: Understanding Immune System in Nursing Module 04: Vaccine and Vaccination Module 05: Types of Vaccine Module 06: Vaccine Safety Module 07: Working in Different Healthcare Settings Module 08: Effective Communication in Nursing Module 09: Health and Safety in Nursing Module 10: Hygiene in Nursing Module 11: Infection Control Course 04: COVID-19 Critical Care Module 01: Coronavirus- The Outbreak Module 02: Deteriorating Patient Module 03: Day-to-Day Critical Care Module 04: COVID-19 Critical Illness: Clinical Overview Module 05: Treatment Support Module 06: Vaccine for COVID-19 Module 07: Caring Human Factors: Understanding and Applying Module 08: Quarantine Rules and Guidelines Course 05: Post COVID Mental Aid Course Module 01: An Overview of COVID-19 Module 02: COVID-19 Impacts on Mental Health for Children Module 03: COVID-19's Effects on the Mental Health of the Young Generation Module 04: COVID-19's Effects on the Mental Health of the Old Generations Module 05: WHO's Psychological Aid Guidelines for Dealing with Mental Health Issues Caused by the Pandemic Course 06: Medical Laboratory Assistant Training Level 3 Module 01 : Introduction to Microbiology Module 02 : Cell Structure and Organisation Module 03 : Microbial Diversity Prokaryote Diversity Module 04 : Microbial Diversity Eukaryote Diversity Module 05 : Viruses, Viroid's, and Prions Module 06 : Interactions between Microbes and Humans Module 07 : Human Microbial Diseases Module 08 : Microorganisms in the Environment Module 09: Laboratory Equipment's (Part-01) Module 10: Laboratory Equipment's (Part-02) Module 11: Solutions Used in Clinical/Medical Laboratory Module 12: Sterilisation, Disinfection and Decontamination Module 13: Laboratory Accidents and Safety Course 07: Biochemistry Module 01: Introduction to Biochemistry Module 02: Introduction to Cells Module 03: Organic Chemistry Module 04: Biomolecules Module 05: Protein Module 06: Biomolecules of Nucleic Acids Module 07: Genetics Module 08: Metabolism Course 08: Sterile Services Technician Training Level 4 Introduction Universal Precautions The Laminar Air Flow Hood (LAF) 3 lectures Sterile Compounding Pharmaceuticals Demonstrations Compounding Pharmacy Math BONUS REVIEW OF THE STERILE COMPOUNDING PROCESS Course 09: Infection Prevention Training Module 01: Infection Prevention and Control Policy in the UK Module 02: Principles of Prevention and Control of Infection Module 03: Immunization Module 04: Infections Spread by Food and Water Module 05: Infections Spread by Animals and Insects, and Less Common Infections Found in the UK Module 06: Infections & Diseases Spread by Person-to-Person Contact Module 07: Infections Spread by Sexual Contact Module 08: Infections Spread by Blood and Body Fluids Course 10: Coronavirus (COVID-19) Awareness Introduction to COVID-19 Understanding of Covid-19 as a Pandemic and How It Is Related to Zoonotic Diseases Like SARS and MERS The Strike of Pandemic Around the World and Great Britain Effects of Covid-19 on Human Life Diagnosis and Treatment Preventions and Social Measures to Be Taken Artificial Intelligence, Data Science and Technological Solutions against Covid-19 Course 11: Personal Hygiene Module 01: Introduction to Personal Hygiene and a Brief History Module 02: Looking Clean, Smelling Clean Module 03: Improving and Maintaining Oral Health Module 04: Care of the Eyes Module 05: Ear Care Secrets Module 06: Foot & Nail Care in Personal Hygiene Module 07: Hair Care Hygienic Ways Module 08: Hygienic Approach to Skin Care Module 09: Maintain Good Toilet Hygiene Module 10: Potential Hygiene Guidelines Module 11: Personal Hygiene During Coronavirus Pandemic CPD 120 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Anyone from any background can enrol in this Epidemiology: Understanding the Spread and Control of Diseases bundle. Requirements This Epidemiology: Understanding the Spread and Control of Diseases course has been designed to be fully compatible with tablets and smartphones. Career path Having this expertise will increase the value of your CV and open you up to multiple job sectors. Certificates Certificate of completion Digital certificate - Included

Best Price Of The Market 'Don't sell yourself short' - The HealthCare Management Training course is intended not only for specialists or professionals but also for anyone with no prior experience. The only thing you will need is the willingness to boost your career with Apex Learning and get advanced in the field of the health industry. Healthcare services are not restricted to patient consideration only. Whether you are running your own training or overseeing others in a centre or office, you should have the option to lead individuals around you and grasp the monetary scene. You should have the ability to establish financial plans and explore the administrative climate. All of this will be available to you through The HealthCare Management Training course designed by specialists of the industry. The HealthCare Management program is pointed toward giving anyone a professional development set to get noticed by recruiters this hiring season. This course focuses on how to work on your skill as an individual in your picked field while additionally illustrating fundamental vocation experiences in this job sector. Hence, you can build up your own expertise and fundamental information, which will help you in arriving at your goal. Further, this course will enhance your resume to stand apart as a potential employer. The best part of this course is you won't have to have any prior experience. You can start from zero learning from the basics to the advanced areas of health management. Along with this HealthCare Management course, you will get 10 premium courses, an originalHardcopy, 11 PDF Certificates (Main Course + Additional Courses) Student ID card as gifts. This HealthCare Management Bundle Consists of the following Premium courses: Course 01: Working in Healthcare Setting Level 3 Course 02: Control and Administration of Medicines Online Training Course Course 03: Pharmacology Course 04: Biomedical Science Course 05: Vaccine Management Course 06: Public Health Course 07: Epidemiology Course 08: Coronavirus (COVID-19) Awareness Course 09: GDPR in Healthcare Course 10: Medical Law Course 11: European Medical Device Regulations So, enrol now to advance your career! Benefits you'll get choosing Apex Learning for this HealthCare Management: Pay once and get lifetime access to 11 CPD courses Free e-Learning App for engaging reading materials & helpful assistance Certificates, student ID for the title course included in a one-time fee Free up your time - don't waste time and money travelling for classes Accessible, informative modules designed by expert instructors Learn at your ease - anytime, from anywhere Study the course from your computer, tablet or mobile device CPD accredited course - improve the chance of gaining professional skills Gain valuable knowledge without leaving your home How will I get my Certificate? After successfully completing the course you will be able to order your CPD Accredited Certificates (PDF + Hard Copy) as proof of your achievement. PDF Certificate: Free (Previously it was £6*11 = £66) Hard Copy Certificate: Free (For The Title Course: Previously it was £10) PS The delivery charge inside the UK is £3.99, and international students have to pay £9.99. **Curriculum** Course 01: Working in Healthcare Setting Level 3 Module 1: Introduction Module 2: Communication and its Relevance Module 3: Rights and Responsibilities as a Health and Social Care Worker Module 4: Role as A Caregiver and Healthcare Professional Module 5: Working in Health and Social Care; Promoting Equality, Diversity and Rights Module 6: Important Principles and Policies in Health and Social Care Work Module 7: Understanding Legal, Professional Standards of Practice and Ethical Aspects of Health Care Part - 1 Module 8: Understanding Legal, Professional Standards of Practice and Ethical Aspects of Health Care Part - 2 Module 9: Safeguarding Vulnerable Individuals Module 10: Health and Safety Responsibilities Module 11: Risk Management in Health and Social Care Course 02: Control and Administration of Medicines Online Training Course Module 01: Medicine Management Fundamentals Module 02: Importance of Medicine Management Module 03: Prescribing Medication Effectively Module 04: Common Problems - Pharmacological Management Module 05: Common Medicines to Use Module 06: Palliative Care Module 07: The Four Principles of Medicine Optimisation Module 08: Managing Medication in Residential Aged Care Facilities (RACFs) Module 09: Law and Legislation Course 03: Pharmacology Module 1: Fundamental Principles of Pharmacology Module 2: Drug Development and Regulation Module 3: Neuropharmacology Module 4: Cardiovascular Pharmacology Module 5: Endocrine Pharmacology Module 6: Chemotherapy Module 7: Inflammation and Immune Pharmacology Module 8: Toxicology Course 04: Biomedical Science Module 01: Introduction to Biomedical Science Module 02: Genetics and Biochemistry Module 03: Microbiology and Cell Biology Module 04: Biochemical Engineering and Enzyme Discovery Module 05:Toxicology, Pharmaceuticals and Fine Chemicals Module 06: Systems and Synthetic Biology Module 07: Global Health Challenges Course 05: Vaccine Management Module 1: Introduction to Vaccine and Vaccination Module 2: Types of Vaccine Module 3: Immunology and Immunisation Module 4: Vaccine Safety Module 5: Communication Module 6: Vaccines in Pandemic Module 7: Paediatric Vaccination Course 06: Public Health Module 01: Introduction to Public Health Module 02: Principles of Public Health Module 03: Understanding Epidemiology Module 04: Disease Control Module 05: Understanding Measures of Disease Frequency Module 06: Maternity and Childbirth Module 07: Environment and Public Health Module 08: Health System and Policy Module 09: Public Health and Ethics Course 07: Epidemiology Module 01: Understanding Epidemiology Module 02: Principles of Epidemiology Module 03: Study Design Module 04: Disease Occurrence Module 05: Variation in Disease by Time, Place, and Person Module 06: Epidemics and Outbreaks Module 07: Measures of Risk and Disease Prevention Module 08: Measures of Disease Frequency Module 09: Role of Epidemiology in Public Health Module 10: The Epidemiological Concept of Population Course 08: Coronavirus (COVID-19) Awareness Introduction to COVID-19 Understanding of Covid-19 as a Pandemic and How It Is Related to Zoonotic Diseases Like SARS and MERS The Strike of Pandemic Around the World and Great Britain Effects of Covid-19 on Human Life Diagnosis and Treatment Preventions and Social Measures to Be Taken Artificial Intelligence, Data Science and Technological Solutions against Covid-19 Course 09: GDPR in Healthcare Module 01: Introduction to GDPR Module 02: GDPR and Healthcare Setting Module 03: General Data Protection Regulations Explained Module 04: Lawful Basis for Preparation Module 05: Responsibilities and Obligations Module 06: Electronic Medical Records Module 07: Rights and Breaches Course 10: Medical Law Module 01- An Introduction to Medical Law Module 02- Legislation on Access to Health, Medical Report, Treatment Module 03- Legislation on Adult Support Module 04- Legislation on Public Health and Health Service (Part 1) Module 05- Legislation on Public Health and Health Service (Part 2) Module 06- Legislation on Public Health and Health Service (Part 3) Module 07- Legislation on Public Health and Health Service (Part 4) Module 08- Legislation on Coronavirus Module 09- Legislation on Mental Health (Part 1) Module 10- Legislation on Mental Health (Part 2) Module 11- Legislation on Abortion Module 12- Other Legislation (Part 1) Module 13- Other Legislation (Part 2) Course 11: European Medical Device Regulations Module 1: Overview to European Regulations on Medical Devices Module 2: Essential Components of EU MDR Module 3: Reporting Requirements and Identification Module 4: Quality System in Medical Device Regulation CPD 110 CPD hours / points Accredited by CPD Quality Standards Who is this course for? There is no experience or previous qualifications required for enrolment on this course. It is available to all students, of all academic backgrounds. Requirements This training is fully compatible with PC's, Mac's, Laptop, Tablet and Smartphone devices. This course has been designed to be fully compatible on tablets and smartphones so you can access your course on wifi, 3G or 4G. Career path Having this CPD certificate will increase the value in your CV and open you up to multiple sectors Certificates Certificate of completion Digital certificate - Included Certificate of completion Hard copy certificate - Included You will get the Hard Copy certificate for the title course (Working in Healthcare Setting Level 3) absolutely Free! Other Hard Copy certificates are available for £10 each. Please Note: The delivery charge inside the UK is £3.99, and the international students must pay a £9.99 shipping cost.

------------------------NEW YEAR SALE ENDS TODAY------------------------ Boost Your Career with Apex Learning and Get Noticed By Recruiters in this Hiring Season! Save Up To £4,169 and get Hard Copy + PDF Certificates + Transcript + Student ID Card worth £160 as a Gift - Enrol Now Give a compliment to your career and take it to the next level. This Clinical Observations Skills will provide you with the essential knowledge and skills required to shine in your professional career. Whether you want to develop skills for your next job or want to elevate skills for your next promotion, this coursewill help you keep ahead of the pack. The Clinical Observations Skills incorporates basic to advanced level skills to shed some light on your way and boost your career. Hence, you can reinforce your professional skills and knowledge, reaching out to the level of expertise required for your position. Further, this coursewill add extra value to your resume to stand out to potential employers. Throughout the programme, it stresses how to improve your competency as a person in your profession while at the same time it outlines essential career insights in this job sector. Consequently, you'll strengthen your knowledge and skills; on the other hand, see a clearer picture of your career growth in future. By the end of the Clinical Observations Skills, you can equip yourself with the essentials to keep you afloat into the competition. Along with thiscourse, you will get 10 other premium courses. Also, you will get an original Hardcopy and PDF certificate for the title course and a student ID card absolutely free. What other courses are included with this Clinical Observations Skills? Course 1: Clinical Governance - Level 3 Course 2: CPD Accredited - CPR and Paediatric First Aid Course Course 3: Sterile Services Technician Training Level 4 Course 4: Human Anatomy and Physiology Course 5: Diabetes Care Diploma Course 6: Medication Administration Level 4 Course 7: Phlebotomy Diploma Course 8: Palliative and End of Life Care Training- Level 5 Course 9: Personal Hygiene Course 10: Mental Health Course As one of the top course providers in the UK, we're committed to providing you with the best educational experience possible. Our industry experts have designed the Clinical Observations Skills to empower you to learn all at once with accuracy. You can take the course at your own pace - anytime, from anywhere. So, enrol now to advance your career! Benefits you'll get choosing Apex Learning for this Clinical Observations Skills: One payment, but lifetime access to 11 CPD courses Certificate, student ID for the title course included in a one-time fee Full tutor support available from Monday to Friday Free up your time - don't waste time and money travelling for classes Accessible, informative modules taught by expert instructors Learn at your ease - anytime, from anywhere Study the course from your computer, tablet or mobile device CPD accredited course - improve the chance of gaining professional skills Gain valuable and in-demand skills without leaving your home. How will I get my Certificate? After successfully completing the course you will be able to order your CPD Accredited Certificates (PDF + Hard Copy) as proof of your achievement. PDF Certificate: Free (Previously it was £10 * 11 = £110) Hard Copy Certificate: Free (For The Title Course) If you want to get hardcopy certificates for other courses, generally you have to pay £20 for each. But this Fall, Apex Learning is offering a Flat 50% discount on hard copy certificates, and you can get each for just £10! P.S. The delivery charge inside the U.K. is £3.99 and the international students have to pay £9.99. Curriculum of Clinical Observations Skills Bundle Course 1: Clinical Observations Skills Module 01: Introduction Module 02: Legal Standards and Professional Aspects of Ethical Practice Module 03: Patient's Condition Observation Module 04: Clinical Management Observation Module 05: Technical Clinical Method Observation Module 06: Medicine and Dispensaries Observation Module 07: Health Safety and Hygiene Course 2: Clinical Governance - Level 3 Module 01: Introduction Module 02: The 7 Pillars of Clinical Governance Module 03: National Health Services Module 04: Clinical Effectiveness: Part 1 Module 05: Clinical Effectiveness: Part 2 Module 06: Clinical Audit Module 07: Confidentiality in a Medical Environment Module 08: Information Technology in Health Care Module 09: Legal aspects and Ethics Module 10: Maintaining Workplace Safety Module 11: Covid-19 Awareness Course 3: CPD Accredited - CPR and Paediatric First Aid Course Module 01: Introduction to Paediatric First Aid Module 02: Assessment and Assistance Module 03: Loss of Responsiveness and CPR Module 04: Paediatric First Aid for Common Illnesses, Injuries and Incidents Module 05: First Aid for Acute Medical Conditions Module 06: Call for an Emergency Course 4: Sterile Services Technician Training Level 4 Introduction Universal Precautions The Laminar Air Flow Hood (LAF) 3 lectures Sterile Compounding Pharmaceuticals Demonstrations Compounding Pharmacy Math BONUS REVIEW OF THE STERILE COMPOUNDING PROCESS Course 5: Human Anatomy and Physiology Human Anatomy and Physiology Module 01: Human Body Vocabulary with Pictures Module 02: Equipment, Instruments and Lab Tests Module 03: Acronyms and Abbreviation Module 04: Digestive System Module 05: Cardiovascular System Module 06: Blood and Immunity System Module 07: Respiratory System Module 08: Musculoskeletal System Module 09: Central Nervous System Course 6: Diabetes Care Diploma Module 01: What is Diabetes Module 02: The Diabetes Challenge Module 03: The Cost of Diabetes in Social Care Module 04: Type 1 Diabetes Module 05: Type 2 Diabetes Module 06: Type 2 Diabetes Treatments Module 07: Prediabetes Module 08: Gestational Diabetes Module 09: Other Types of Diabetes Module 10: Hypoglycaemia Module 11: Hyperglycaemia and Hyperosmolar Hyperglycaemic State Module 12: Glossary of Diabetes Terms Course 7: Medication Administration Level 4 Module 01: Introduction to Safe Handling of Medicines Module 02: Legislation and guidance of medication management Module 03: Principles of Safe and Appropriate Handling of Medicines Module 04: Medication Risk Assessment Guidance Module 05: Handling Medicines in Social Care Settings Module 06: Consent to Treatment Module 07: Requirements for Specific Services Module 08: Levels of Care and Support Module 09: Procedure for Handling Medication Module 10: The Six Rights of Medication Administration Module 11: Covert Administration of Medication Module 12: Recording Procedures Module 13: Storage of Medication Module 14: Transfer and Disposal of Medication Module 15: Drug Formulation and Ways of Taking It Course 8: Phlebotomy Diploma Module 01: Introduction to Phlebotomy Module 02: Blood Circulation, Function, and Composition Module 03: Phlebotomy Equipment Module 04: Routine Venipuncture Module 05: Venipuncture Complications and Pre-Examination Variables Module 06: Dermal Puncture Module 07: Quality Assessment and Management in Phlebotomy Module 08: Special Blood Collection Procedure Module 09: Infection Control and Risk Management Course 9: Palliative and End of Life Care Training- Level 5 Module 1: Introduction to Palliative & End of Life Care Module 2: Identification of the Stage of Illness Module 3: Palliative Care Holistic Assessment Module 4: Advance Care Planning Module 5: Care at the End of Life Module 6: Care after Death Module 7: Further Support, Training and Development Module 8: Legal Issues Module 9: Glossary of Terms Module 10: Appendices Course 10: Personal Hygiene Module 01: Introduction to Personal Hygiene and a Brief History Module 02: Looking Clean, Smelling Clean Module 03: Improving and Maintaining Oral Health Module 04: Care of the Eyes Module 05: Ear Care Secrets Module 06: Foot & Nail Care in Personal Hygiene Module 07: Hair Care Hygienic Ways Module 08: Hygienic Approach to Skin Care Module 09: Maintain Good Toilet Hygiene Module 10: Potential Hygiene Guidelines Module 11: Personal Hygiene During Coronavirus Pandemic Course 11: Mental Health Course Module 01: Introduction to Mental Health Module 02: Childhood And Adolescent Psychiatric Disorders Module 03: Stress and Anxiety Disorders Module 04: Schizophrenia Module 05: Personality Disorders Module 06: Mood Disorders Module 07: Eating and Sleeping Disorders Module 08: Self-Harm and Suicide Module 09: Medication and Therapy in the Treatment of Mental Illness Module 10: Social Attitudes To Mental Illness Module 11: Mental Health Legislation and Services Module 12: Mental Health In The Workplace CPD 110 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Anyone from any background can enrol in this Clinical Observations Skills bundle. Persons with similar professions can also refresh or strengthen their skills by enrolling in this course. Students can take this course to gather professional knowledge besides their study or for the future. ** Completing this course does not imply that you are qualified to work as a Clinical Observer. If you are seeking a course that fits the HCPC's standards, please contact the HCPC or the RCSLT ** Requirements Our Clinical Observations Skills is fully compatible with PC's, Mac's, Laptop, Tablet and Smartphone devices. This course has been designed to be fully compatible with tablets and smartphones so you can access your course on Wi-Fi, 3G or 4G. There is no time limit for completing this course, it can be studied in your own time at your own pace. Career path Having these various certifications will increase the value in your CV and open you up to multiple job sectors. Certificates Certificate of completion Digital certificate - Included

Boost Your Career with Apex Learning and Get Noticed By Recruiters in this Hiring Season! Save Up To £4,169 and get Hard Copy + PDF Certificates + Transcript + Student ID Card worth £160 as a Gift - Enrol Now If you've ever been fascinated by the world of healthcare and wanted to play a crucial role in ensuring the well-being of patients, then the Sterile Services Technician Training Level 4 bundle is your calling. This bundle contains 11 courses that will equip you with the knowledge and skills needed to excel in your career and make a real difference in the lives of those you care for. At the heart of this bundle lies the Sterile Services Technician Training Level 4 course, which will take you on a deep dive into the world of sterile services. You'll learn about the latest sterilisation techniques, equipment, and regulations and discover how to manage and maintain a sterile services department. Armed with this knowledge, you'll be able to make a significant impact on patient safety and quality of care in your workplace. And with a hardcopy CPD certificate included in the course, you'll have tangible proof of your expertise and dedication. But that's not all. The bundle also includes 10 additional courses covering a range of topics, from phlebotomy and medication administration to GDPR and infection prevention. Each course is CPD certified, so you can be sure that the knowledge you gain is of the highest quality. Join the ranks of the unsung heroes of healthcare. Enrol in the Sterile Services Technician Training Level 4 bundle and start making a difference today. Learning outcomes for the Sterile Services Technician Training Level 4 bundle: Develop a deep understanding of sterile services and infection prevention Learn the latest techniques and regulations for sterilising medical equipment Master medication administration and phlebotomy skills Understand the principles of GDPR in healthcare Acquire knowledge of palliative and end-of-life care Learn about adult nursing and community practice nursing Enhance your understanding of human anatomy and physiology Gain knowledge of consent in healthcare Be a superhero, staying beyond clean, with the Sterile Services Technician Training Level 4 course. Courses are included in this Sterile Services Technician Training Level 4: Course 1: Sterile Services Technician Training Level 4 Course 2: Phlebotomy Diploma Course 3: Medication Administration Level 4 Course 4: Health and Social Care UK 2021 Course 5: Palliative and End of Life Care Training- Level 5 Course 6: Consent in Healthcare Course 7: Human Anatomy and Physiology Course 8: Adult Nursing Diploma Course 9: Community Practice Nurse Course 10: GDPR in Healthcare Course 11: Infection Prevention Training Benefits you'll get choosing Apex Learning for this Sterile Services Technician Training Level 4: One payment, but lifetime access to 11 CPD courses Certificates, student ID for the title course included in a one-time fee Full tutor support available from Monday to Friday Free up your time - don't waste time and money travelling for classes Accessible, informative modules taught by expert instructors Learn at your ease - anytime, from anywhere Study the course from your computer, tablet or mobile device CPD accredited course - improve the chance of gaining professional skills Gain valuable knowledge without leaving your home Curriculum of Sterile Services Technician Training Level 4 Bundle Course 1: Sterile Services Technician Training Level 4 Introduction Universal Precautions The Laminar Air Flow Hood (LAF) 3 lectures Sterile Compounding Pharmaceuticals Demonstrations Compounding Pharmacy Math BONUS REVIEW OF THE STERILE COMPOUNDING PROCESS Course 2: Phlebotomy Diploma Module 01: Introduction to Phlebotomy Module 02: Blood Circulation, Function, and Composition Module 03: Phlebotomy Equipment Module 04: Routine Venipuncture Module 05: Venipuncture Complications and Pre-Examination Variables Module 06: Dermal Puncture Module 07: Quality Assessment and Management in Phlebotomy Module 08: Special Blood Collection Procedure Module 09: Infection Control and Risk Management Course 3: Medication Administration Level 4 Module 01: Introduction to Safe Handling of Medicines Module 02: Legislation and guidance of medication management Module 03: Principles of Safe and Appropriate Handling of Medicines Module 04: Medication Risk Assessment Guidance Module 05: Handling Medicines in Social Care Settings Module 06: Consent to Treatment Module 07: Requirements for Specific Services Module 08: Levels of Care and Support Module 09: Procedure for Handling Medication Module 10: The Six Rights of Medication Administration Module 11: Covert Administration of Medication Module 12: Recording Procedures Module 13: Storage of Medication Module 14: Transfer and Disposal of Medication Module 15: Drug Formulation and Ways of Taking It Course 4: Health and Social Care UK 2021 Module 01: Introduction to Advanced Health and Social Care Module 02: Introduction to the UK Health System Module 03: Essential Principles, Policies and Legislations in Health and Social Care Work Module 04: Fundamental Aspects of Providing Quality Health Course 5: Palliative and End of Life Care Training- Level 5 Module 1: Introduction to Palliative & End of Life Care Module 2: Identification of the Stage of Illness Module 3: Palliative Care Holistic Assessment Module 4: Advance Care Planning Module 5: Care at the End of Life Module 6: Care after Death Module 7: Further Support, Training and Development Module 8: Legal Issues Module 9: Glossary of Terms Module 10: Appendices Course 6: Consent in Healthcare ***Covid-19 Awareness for General People and Healthcare*** Module 01: Introduction to Covid-19 Module 02: Understanding of Covid-19 as a Pandemic and How It Is Related to Zoonotic Diseases Like SARS and MERS Module 03: The Strike of Pandemic Around the World and Great Britain Module 04: Effects of Covid-19 on Human Life Module 05: Diagnoses and Treatments Module 06: Preventions and Social Measures to Be Taken Module 07: Artificial Intelligence, Data Science and Technological Solutions against Covid-19 ***Consent Training in Healthcare Course*** Module 01: An Introduction to Consent Module 02: Information Regarding Consent Module 03: Consent Training in Healthcare Course Module 04: Best Interest Decision Making Module 5: Assessing Capacity Module 06: Providing Care or Treatment to People Who Lack Capacity Module 07: Public Bodies and Services Created by MCA Act 2005 Module 08: UK Acts in Relation to Consent Module 09: Handling Complaints Regarding Consent Course 7: Human Anatomy and Physiology Module 01: Human Body Vocabulary with Pictures Module 02: Equipment, Instruments and Lab Tests Module 03: Acronyms and Abbreviation Module 04: Digestive System Module 05: Cardiovascular System Module 06: Blood and Immunity System Module 07: Respiratory System Module 08: Musculoskeletal System Module 09: Central Nervous System Course 8: Adult Nursing Diploma An Introduction to Safeguarding of Vulnerable Adults Safeguarding Legislations Understanding Abuse and Neglect Safeguarding Vulnerable Adults Policy, Roles and Responsibilities Understanding the Importance of Communication in Care Sharing Information and Responding To a Disclosure Reporting Your Concern Course 9: Community Practice Nurse Module 01 : Role as A Caregiver and Healthcare Professional Module 02 : Introduction to Nursing Module 03 : Working in Different Healthcare Settings Module 04 : Effective Communication in Nursing Module 05 : Health and Safety in Nursing Module 06 : Hygiene in Nursing Module 07 : Infection Control Module 08 : Understanding the Immune System in Nursing Module 09 : Rest and Sleep Management in Nursing Module 10 : Pain Management for Nurses Module 11 : Nutrition in Nursing Module 12 : Fluid and Electrolyte Balance Module 13 : Assisting with Elimination Module 14 : Oxygenation in Nursing Module 15 : Risk Management in Health and Social Care Course 10: GDPR in Healthcare Module 01: Introduction to GDPR Module 02: GDPR and Healthcare Setting Module 03: General Data Protection Regulations Explained Module 04: Lawful Basis for Preparation Module 05: Responsibilities and Obligations Module 06: Electronic Medical Records Module 07: Rights and Breaches Course 11: Infection Prevention Training Module 01: Infection Prevention and Control Policy in the UK Module 02: Principles of Prevention and Control of Infection Module 03: Immunization Module 04: Infections Spread by Food and Water Module 05: Infections Spread by Animals and Insects, and Less Common Infections Found in the UK Module 06: Infections & Diseases Spread by Person-to-Person Contact Module 07: Infections Spread by Sexual Contact Module 08: Infections Spread by Blood and Body Fluids How will I get my Certificate? After successfully completing the course you will be able to order your CPD Accredited Certificates (PDF + Hard Copy) as proof of your achievement. PDF Certificate: Free (Previously it was £6*11 = £66) Hard Copy Certificate: Free (For The Title Course: Previously it was £10) P.S. The delivery charge inside the U.K. is £3.99 and the international students have to pay £9.99. CPD 115 CPD hours / points Accredited by CPD Quality Standards Who is this course for? There is no experience or previous qualifications required for enrolment on this Sterile Services Technician Training Level 4. It is available to all students, of all academic backgrounds. Requirements Our Sterile Services Technician Training Level 4 is fully compatible with PC's, Mac's, Laptop, Tablet and Smartphone devices. This course has been designed to be fully compatible on tablets and smartphones so you can access your course on wifi, 3G or 4G. There is no time limit for completing this course, it can be studied in your own time at your own pace. Career path Having this CPD certificate will increase the value in your CV and open you up to multiple sectors such as Business & Management, Admin, Accountancy & Finance, Secretarial & PA, Teaching & Mentoring etc. Certificates Certificate of completion Digital certificate - Included Certificate of completion Hard copy certificate - Included You will get the Hard Copy certificate for the title course (Sterile Services Technician Training Level 4) absolutely Free! Other Hard Copy certificates are available for £10 each. Please Note: The delivery charge inside the UK is £3.99, and the international students must pay a £9.99 shipping cost.

Get Hard Copy + PDF Certificates + Transcript + Student ID Card worth £160 as a Gift - Enrol Biomedical Science & Biomedical Engineering Diploma Now COVID-19 is the recent pandemic that wreaked havoc worldwide and could have caused more causalities if not for Biomedical Science. In addition, the vaccines that were invented were based on Biomedical Science research. Not only that, Biomedical science is important because it is the first step toward the creation of new medications and treatments that help manage all different types of health conditions and diseases. Without Biomedical Science, the prevention and cure of diseases would be practically impossible. The employment of biomedical engineers is projected to grow by 7% from 2016 to 2026. Biomedical engineers will likely see employment growth because of the increasing possibilities of new technologies and rising applications to medical equipment and devices. And starting salaries range from £24,214 to £30,112 in Biomedical Science jobs, and with experience and/or specialist knowledge; you can earn a wage of £30,401 to £37,005. Along with this Biomedical Science & Biomedical Engineering Diploma course, you will get 10 premium courses, an original Hardcopy, 11 PDF Certificates (Main Course + Additional Courses) Student ID card as gifts. This Biomedical Science & Biomedical Engineering Bundle Consists of the following Premium courses: Course 01: Biomedical Science Course 02: Biochemistry Course 03: Diploma In Microbiology Course 04: Biotechnology Course 05: Microbiology Technician Course Course 06: Lab Analyst Training Course 07: Medication Administration Level 4 Course 08: GDPR in Healthcare Course 09: Public Health Course 10: Epidemiology Course 11: Pandemic Disease Awareness Key features of this Biomedical Science & Biomedical Engineering: This bundle is CPD QS Accredited Learn from anywhere in the world Lifetime access Biomedical Science & Biomedical Engineering is entirely online 24/7 Learner support So, enrol in this Biomedical Science & Biomedical Engineering Diploma now to advance your career! The Biomedical Science & Biomedical Engineering bundle incorporates basic to advanced level skills to shed some light on your way and boost your career. Hence, you can strengthen your Biomedical Science & Biomedical Engineering expertise and essential knowledge, which will assist you in reaching your goal. Moreover, you can learn from any place in your own time without travelling for classes. Curriculum of Biomedical Science & Biomedical Engineering Diploma Course 01: Biomedical Science Introduction to Biomedical Science Genetics and Biochemistry Microbiology and Cell Biology Biochemical Engineering and Enzyme Discovery Toxicology, Pharmaceuticals and Fine Chemicals Systems and Synthetic Biology Global Health Challenges ----------- 10 more Biomedical Science & Biomedical Engineering Related Courses------------ How will I get my Biomedical Science & Biomedical Engineering Diploma Certificate? After successfully completing the Biomedical Science & Biomedical Engineering Diploma, you will be able to order your CPD Accredited Certificates (PDF + Hard Copy) as proof of your achievement. PDF Certificate: Free (Previously it was £6*11 = £66) Hard Copy Certificate: Free (For The Title Course: Previously it was £10) CPD 110 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Anyone from any background can enrol in this Biomedical Science & Biomedical Engineering Diploma bundle. Requirements This Biomedical Science & Biomedical Engineering Diploma has been designed to be fully compatible with tablets and smartphones. Career path This Biomedical Science & Biomedical Engineering Diploma Bundle is would be beneficial for any related profession or industry such as: Analytical Chemists Lab Technicians Biomedical Researchers Scientists Clinical Scientists Environmental Engineers Forensic Science Technicians Medicinal Chemists Medical Writers Microbiologists Research Scientists Certificates Certificate of completion Digital certificate - Included Certificate of completion Hard copy certificate - Included You will get the Hard Copy certificate for the title course (Biomedical Science) absolutely Free! Other Hard Copy certificates are available for £10 each. Please Note: The delivery charge inside the UK is £3.99, and the international students must pay a £9.99 shipping cost.

Boost Your Career with Apex Learning and Get Noticed By Recruiters in this Hiring Season! Get Hard Copy + PDF Certificates + Transcript + Student ID Card worth £160 as a Gift - Enrol Now With a single payment you will gain access to Genetics including 10 additional premium courses, original hardcopy certificate, transcript and a student ID card which will allow you to get discounts on things like music, food, travel and clothes etc. Courses are included with this Genetics Complete Bundle: Course 01: Genetics Course 02: Biotechnology Course 03: Biomedical Science Course 04: Human Anatomy and Physiology Course 05: Lab Analyst Training Course 06: Phlebotomy Course 07: Animal Science Course 08: Infection Prevention Training Course 09: Medical Law Course 10: Personal Hygiene Course 11: Coronavirus (COVID-19) Awareness If you want to gain a solid understanding of Genetics and fast track your dream career, then take a step in the right direction with this industry-standard, comprehensive Genetics course designed by expert instructors. The course will cover a comprehensive range of 5, you'll have 24/7 online access to the study materials, introductory videos, assessment tests, study resources and tutor support for a lifetime. The Genetics will help you to develop your skills, confidence and knowledge of this sector, helping to add real value to your CV and personal development. Gain experience online and interact with experts. This can prove to be the perfect way to get noticed by a prospective employer and stand out from the crowd. This course has been rated and reviewed highly by our learners and professionals alike. We have a passion for teaching, and it shows. All of our courses have interactive online modules that allow studying to take place where and when you want it to. The only thing you need to take this course is Wi-Fi and a screen. You'll never be late for class again. Experienced tutors and mentors will be there for you whenever you need them, and solve all your queries through email and chat boxes. Whether you are looking to brighten up your CV, just starting out in the industry, looking for a career change or just fancy learning something new, this online course is perfect! Benefits you'll get choosing Apex Learning: One payment, but lifetime access to 11 CPD courses Certificates, student ID for the title course included in a one-time fee Full tutor support available from Monday to Friday Free up your time - don't waste time and money travelling for classes Accessible, informative modules taught by expert instructors Learn at your ease - anytime, from anywhere Study the course from your computer, tablet or mobile device CPD accredited course - improve the chance of gaining professional skills Gain valuable experience without leaving your home Curriculum Course 01: Genetics Module 01- Fundamental Concepts of Genetics Module 02- Genetics and Genomics Module 03- Genetic Disorders Module 04- Genetic Counselling Module 05- Application of Genetics Knowledge Course 02: Biotechnology Module 1 - Introduction to Biotechnology Module 2- Biotechnology in the Realm of History Module 3- Molecular and Cellular Biology Module 4 - Biotechnology Versus Molecular Biology Module 5- Genetic Engineering Module 6- Biotechnology in Health Sector Module 7- Industrial Biotechnology Module 8- Biotechnology in a Business Perspective Course 03: Biomedical Science Module 01: Introduction to Biomedical Science Module 02: Genetics and Biochemistry Module 03: Microbiology and Cell Biology Module 04: Biochemical Engineering and Enzyme Discovery Module 05:Toxicology, Pharmaceuticals and Fine Chemicals Module 06: Systems and Synthetic Biology Module 07: Global Health Challenges Course 04: Human Anatomy and Physiology Module 01: Human Body Vocabulary with Pictures Module 02: Equipment, Instruments and Lab Tests Module 03: Acronyms and Abbreviation Module 04: Digestive System Module 05: Cardiovascular System Module 06: Blood and Immunity System Module 07: Respiratory System Module 08: Musculoskeletal System Module 09: Central Nervous System Course 05: Lab Analyst Training Introduction to the Course Medical Laboratory Services Laboratory Equipments (Part-01) Laboratory Equipments (Part-02) Microscope and How to Use It Solutions Used in Clinical/Medical Laboratory Sterilisation, Disinfection and Decontamination Basic Haematology Basic Haemostasis (Coagulation) Basic Immunology and Immunohematology Urinalysis Basic Clinical Chemistry Basic Clinical Microbiology Basic Parasitology Laboratory Accidents and Safety Quality Assurance Course 06: Phlebotomy Module 01: Introduction to Phlebotomy Module 02: Blood Circulation, Function, and Composition Module 03: Phlebotomy Equipment Module 04: Routine Venipuncture Module 05: Venipuncture Complications and Pre-Examination Variables Module 06: Dermal Puncture Module 07: Quality Assessment and Management in Phlebotomy Module 08: Special Blood Collection Procedure Module 09: Infection Control and Risk Management Course 07: Animal Science Module 01: Principles of Animal Science Module 02: Animal Anatomy and Physiology Module 03: Cell Biology Module 04: Biochemistry Module 05: Animal Microbiology Module 06: Genetics and Evolution Module 07: Animal Nutrition Module 08: Animal Disease and Medicine Module 09: Animal Behavioural Ecology Module 10: Pet Care Module 11: Wildlife Management Module 12: Animal Welfare Course 08: Infection Prevention Training Module 01: Infection Prevention and Control Policy in the UK Module 02: Principles of Prevention and Control of Infection Module 03: Immunization Module 04: Infections Spread by Food and Water Module 05: Infections Spread by Animals and Insects, and Less Common Infections Found in the UK Module 06: Infections & Diseases Spread by Person-to-Person Contact Module 07: Infections Spread by Sexual Contact Module 08: Infections Spread by Blood and Body Fluids Course 09: Medical Law Module 01- An Introduction to Medical Law Module 02- Legislation on Access to Health, Medical Report, Treatment Module 03- Legislation on Adult Support Module 04- Legislation on Public Health and Health Service (Part 1) Module 05- Legislation on Public Health and Health Service (Part 2) Module 06- Legislation on Public Health and Health Service (Part 3) Module 07- Legislation on Public Health and Health Service (Part 4) Module 08- Legislation on Coronavirus Module 09- Legislation on Mental Health (Part 1) Module 10- Legislation on Mental Health (Part 2) Module 11- Legislation on Abortion Module 12- Other Legislation (Part 1) Module 13- Other Legislation (Part 2) Course 10: Personal Hygiene Module 01: Introduction to Personal Hygiene and a Brief History Module 02: Looking Clean, Smelling Clean Module 03: Improving and Maintaining Oral Health Module 04: Care of the Eyes Module 05: Ear Care Secrets Module 06: Foot & Nail Care in Personal Hygiene Module 07: Hair Care Hygienic Ways Module 08: Hygienic Approach to Skin Care Module 09: Maintain Good Toilet Hygiene Module 10: Potential Hygiene Guidelines Module 11: Personal Hygiene During Coronavirus Pandemic Course 11: Coronavirus (COVID-19) Awareness Introduction to COVID-19 Understanding of Covid-19 as a Pandemic and How It Is Related to Zoonotic Diseases Like SARS and MERS The Strike of Pandemic Around the World and Great Britain Effects of Covid-19 on Human Life Diagnosis and Treatment Preventions and Social Measures to Be Taken Artificial Intelligence, Data Science and Technological Solutions against Covid-19 How will I get my Certificate? After successfully completing the course you will be able to order your CPD Accredited Certificates (PDF + Hard Copy) as proof of your achievement. PDF Certificate: Free (Previously it was £10 * 11 = £110) Hard Copy Certificate: Free (For The Title Course) If you want to get hardcopy certificates for other courses, generally you have to pay £20 for each. But this Fall, Apex Learning is offering a Flat 50% discount on hard copy certificates, and you can get each for just £10! P.S. The delivery charge inside the U.K. is £3.99 and the international students have to pay £9.99. CPD 115 CPD hours / points Accredited by CPD Quality Standards Who is this course for? There is no experience or previous certifications required for enrolment on this Genetics. It is available to all students, of all academic backgrounds. Requirements Our Genetics is fully compatible with PC's, Mac's, Laptop, Tablet and Smartphone devices. This course has been designed to be fully compatible on tablets and smartphones so you can access your course on wifi, 3G or 4G. There is no time limit for completing this course, it can be studied in your own time at your own pace. Career path Having these various CPD certificate will increase the value in your CV and open you up to multiple sectors such as Business & Management, Admin, Accountancy & Finance, Secretarial & PA, Teaching & Mentoring etc. Certificates Certificate of completion Digital certificate - Included

Search By Location
- Pharmaceutical Courses in London
- Pharmaceutical Courses in Birmingham
- Pharmaceutical Courses in Glasgow
- Pharmaceutical Courses in Liverpool
- Pharmaceutical Courses in Bristol
- Pharmaceutical Courses in Manchester
- Pharmaceutical Courses in Sheffield
- Pharmaceutical Courses in Leeds
- Pharmaceutical Courses in Edinburgh
- Pharmaceutical Courses in Leicester
- Pharmaceutical Courses in Coventry
- Pharmaceutical Courses in Bradford
- Pharmaceutical Courses in Cardiff
- Pharmaceutical Courses in Belfast
- Pharmaceutical Courses in Nottingham