- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
4418 Pha courses
TOGAF® EA Course - Foundation and Practitioner (Level 1 and 2)
By Advised Skills
This course is designed to enable candidates to develop the knowledge of the terminology and basic concepts of TOGAF Standard, 10th Edition and principles of Enterprise Architecture. Candidates will also be able to analyze and apply knowledge of TOGAF Standard.

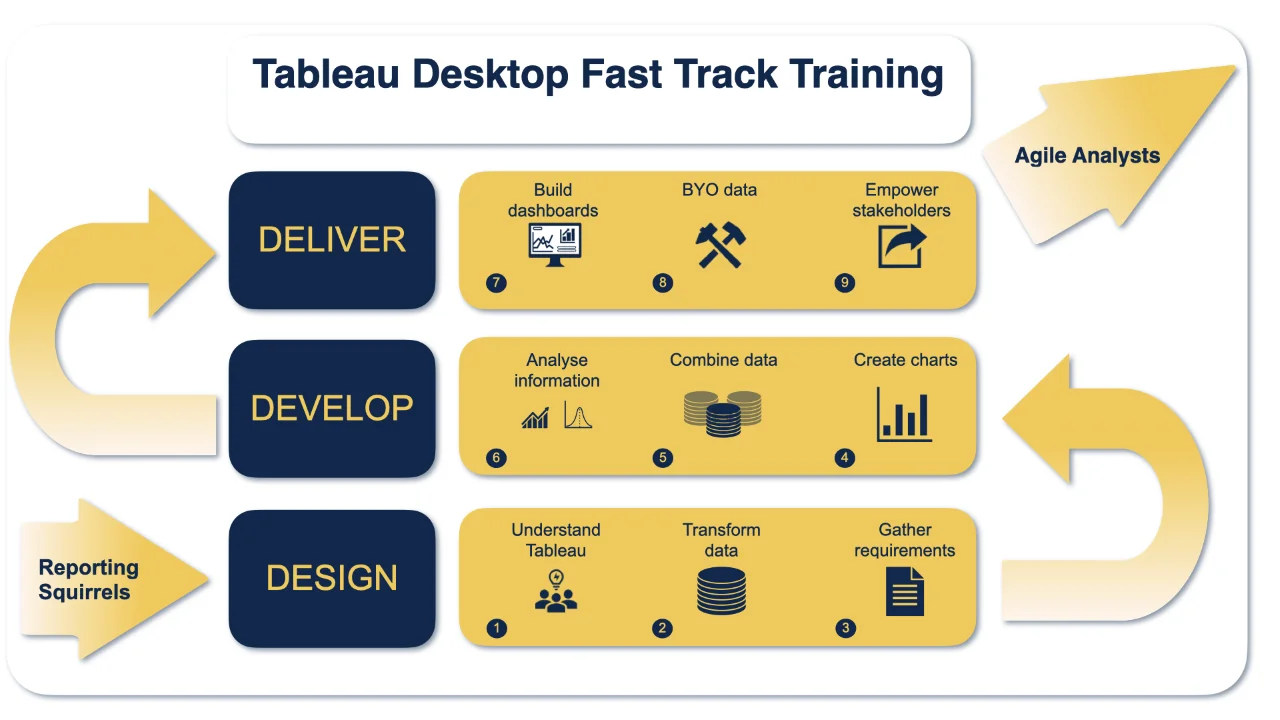
Tableau Training On Tap - Fast Track
By Tableau Training Uk
Tableau is an intuitive and simple tool to learn. This Tableau training course is a jumpstart to getting report writers and analysts who are self-taught or have no previous knowledge to being productive. It covers everything from connecting to data, through to creating interactive dashboards with a range of visualisations in three days. Having a quick turnaround from starting to use Tableau, to getting real, actionable insights means that you get a swift return on your investment. At the end of this course, you will be able to communicate insights more effectively, enabling your organisation to make better decisions, quickly. This accelerated approach is key to getting engagement from within your organisation so everyone can immediately see and feel the impact of the data and insights you create. Our Tableau Desktop Fast Track course combines all of our Foundation (Fundamentals) and Analyst (Intermediate) content into a 3 day live online course with added access to online bonus content of 3 additional modules. Gathering Requirements, Bring Your Own Data and Engaging Users. What do you get? This course is delivered live virtually and has all material provided through our online portal, together with email support and live coaching sessions. The full program includes all of the following elements: 3 days of live and interactive instructor-led sessions delivered by an expert Tableau Trainer 6 weeks access to our live coaching program delivered by expert Tableau coaches 50+ practical exercises to practice what you learn 12 months access to video’s that walk you through the theory and exercise solutions Practical advice, tools and resources for using Tableau in the real world The three additional online modules provide:Clarity on the approach to gathering dashboard requirements in a way that can be translated into dashboard designs.An agile and iterative development process that delivers products that meet user needs more quickly and effectively.An understanding of how end users will interact with dashboards to ensure that designers deliver actionable results. THE SYLLABUS PHASE 1: DESIGN MODULE 1: UNDERSTAND TABLEAU What is possible How does Tableau deal with data Know your way around Review of Type Conversions How do we format charts Dashboard basics – My first Dashboard MODULE 2: TRANSFORM DATA Connecting to and setting up data in Tableau Modifying data attributes How Do I Structure my Data – Groups & Hierarchies, Visual Groups How Tableau Deals with Dates – Using Discrete and Continuous Dates, Custom Dates How do I create calculated fields and why? – Creating Calculated Fields, Types of calculated fields, Row Level v Aggregations, Aggregating dimensions in calculations, Changing the Level of Detail (LOD) of calculations – What, Why, How MODULE 3: GATHER REQUIREMENTS(ONLINE CONTENT ONLY) Brainstorm and assess possible priorities Pitfalls to avoid Gather requirements PHASE 2: DEVELOP MODULE 4: CREATE CHARTS Charts that Compare Multiple Measures – Measure Names and Measure Values, Shared Axis Charts, Dual Axis Charts, Scatter Plots Showing progress over time Creating Tables – Creating Tables, Highlight Tables, Heat Maps Showing Relational & Proportional Data – Pie Charts, Donut Charts, Tree Maps Making things dynamic with parameters MODULE 5: COMBINE DATA Relationships Joining Tables – Join Types, Joining tables within the same database, cross database joins, join calculations Blending – How to create a blend with common fields, Custom defined Field relationships and mismatched element names, Calculated fields in blended data sources Unions – Manual Unions and mismatched columns, Wildcard unions Data Extracts – Creating & Editing Data extracts MODULE 6: ANALYSE INFORMATION Table Calculations Sets, Reference Lines, Trends and Forecasting Answering spatial questions – Mapping, Creating a choropleth (filled) map, Using your own images for spatial analysis, Mapping with spatial files Advanced charts Bar in Bar charts Bullet graphs Creating Bins and Histograms Creating a Box & Whisker plot Viz in Tooltips PHASE 3: DESIGN MODULE 7: BUILD DASHBOARDS Using the Dashboard Interface Device layouts Dashboard Actions – Set actions, Parameter actions Viz in Tooltips for Dashboards Dashboard containers – Horizontal & Vertical containers, Hidden containers Navigate between dashboards Telling data driven stories MODULE 8: BRING YOUR OWN DATA Design Best Practices & Resources Wireframe templates Questions Process Start building and testing MODULE 9: EMPOWER STAKEHOLDERS What is Tableau Server Publishing & permissions How can your users engage with content The Tableau ecosystem Review your progress Your next steps HOW MUCH OF YOUR TIME WILL THIS TAKE? Delegates are also provided 6 weeks access to our Tableau Coaching. We run Live Q&A sessions from 4pm-5pm on a Monday (Connecting to Data and Calculated Fields), 2pm-3pm Friday (Creating Charts) & 4pm-5pm Friday (Dashboard Design). The coaching helps delegates to transition from the theory of using Tableau to its practical use. We’d be expecting them to apply the exercises you’ll be doing during the course, onto your own data after the course. In our experience, this is the best way to increase both understanding and long term memory retention. The live coaching also acts as a troubleshooting platform for any practical issues that delegates need to overcome in the real world. Delegates also have 12 months access to all of the training material covered in the course in the form of an online portal (this includes theory videos, exercise solution videos, exercise materials and even quizzes). We have a growing LinkedIn community that delegates are encouraged to join and participate in. We regularly post useful blog posts and additional training that will enhance the Tableau journey and understanding. We help teams using Tableau to transform in the following ways : From a disjointed understanding of Tableau – To being familiar with Tableau terminology and capability From ad-hoc data uploads and error prone calculations – To reusable data connections and robust metrics From disjointed stakeholder questions – To clear and concise requirements that lead to decision making From being unsure how difficult Tableau will be to learn – To being able to develop standard charts and tables in Tableau with dynamic reporting capabilities From manually combining data for each analytical task – To dynamically combining data from multiple tables for analysis From being unsure how to answer analytical questions and what options there are – To being equipped with multiple actionable, dynamic, analytical use cases From not knowing Why, When and How to create Dashboards or Story’s – To being able to combine analysis to answer complex questions and tell data driven stories From using demo data theory – To Delivering value [Answering questions] on their own data From spending lots of time answering colleagues ad-hoc (data) questions – To empowering stakeholders in answering ad-hoc queries and reducing the time to analyse and steer the business
Project Scope and Schedule Management
By Nexus Human
Duration 2 Days 12 CPD hours This course is intended for This class is intended for PMP?s looking to earn PDU's, or for Project Managers looking to learn more about Project Scope and Schedule Management techniques. This course provides participants with tools and techniques to help them determine and deliver products, services, and results that meet requirements, expectations, and deadlines. NOTE: This course will earn you 14 PDUs. 1 - PROJECT SCOPE AND TIME MANAGEMENT OVERVIEW Project Scope Management Overview Project Scope Mgt. Processes and Key Outputs Project Time Management Overview Project Time Mgt. Processes and Key Outputs Case Study Selection Requirements, Scope, and Negotiations 2 - PLAN SCOPE AND SCHEDULE MANAGEMENT Plan Scope and Schedule Mgt. Overview The Scope Management Plan The Requirements Management Plan The Schedule Management Plan 3 - COLLECT REQUIREMENTS Collect Requirements Overview Types of Requirements Effective Product/Solution Requirements Other Stakeholders and Requirements Collect Requirements Techniques Voice of the Customer Requirements Traceability Matrix 4 - DEFINE SCOPE Define Scope Overview Project Scope Statement Sections Create a Project Scope Statement 5 - CREATE WBS Create WBS Overview WBS by Deliverable WBS by Phase When to Subdivide a Work Package Further Effective WBSs General Process for Creating the WBS Create a WBS 6 - DEFINE ACTIVITIES Define Activities Overview Practical Considerations for Defining Activities Activities and Steps Milestones Create Activities and Milestones 7 - SEQUENCE ACTIVITIES Develop Schedule Overview Critical Path Resource Optimization ? Resource Leveling Resource Optimization ? Resource Smoothing Resolve Overallocations Critical Chain Schedule Compression Techniques 8 - ESTIMATE ACTIVITY RESOURCES Estimate Activity Resources Overview Resource Breakdown Structure Estimate Activity Resources Assign Resources to Activities 9 - ESTIMATE ACTIVITY DURATIONS Estimate Activity Durations Overview Expert Judgment Ratio Estimating Analogous Estimating Parametric Estimating Forecasting Three Point Estimating and PERT Estimating Reserves Estimate Activity Durations 10 - DEVELOP SCHEDULE Develop Schedule Overview Critical Path Resource Optimization ? Resource Leveling Resource Optimization ? Resource Smoothing Resolve Overallocations Critical Chain Schedule Compression Techniques 11 - VALIDATE SCOPE Validate Scope Overview Verify Scope Best Practices Create a Scope Validation Form 12 - CONTROL SCOPE AND SCHEDULE Control Scope Overview Control Schedule Overview Control Scope Best Practices Change Control Stakeholders Determine Change Control Stakeholders Change Control Process Change Request Form

IV Sedation Full Course Dentists and Nurses
By UKSedation
UK Sedation & Mellow Dental Presents: The New Starter Sedation Course for Dentists and Dental Nurses With Dr Roy Bennett and Dr Robert Endicott Are you a dentist or Dental Nurse looking to expand your skills and provide a more comfortable experience for your patients? If so, we’ve got the perfect opportunity for you. Introducing our brand-new, comprehensive New Starter Sedation Course. This innovative course is brought to you by two leading names in dental education – UK Sedation and Mellow Dental. Our commitment is to empower you with the skills and confidence to provide effective sedation in your practice. The Starter Sedation Course spans two days, offering a blend of online and face-to-face learning for the best educational experience: 🌐 Day 1 – Online Learning: Dive into the theory of dental sedation from the comfort of your own home. Our expert instructors will guide you through comprehensive video tutorials, live webinars, and interactive quizzes, making learning both easy and engaging! 👥 Day 2 – Face-to-Face Workshop: Put your newfound knowledge into action with a hands-on, practical workshop held at one of our state-of-the-art training facilities. Here, you’ll learn directly from experienced professionals in a safe, supportive environment. Once those two parts are complete, you have all the theory training required to enter the mentoring phase of your training. 🌐 Post course – Exclusive access to our online mentoring and logging programme. You need to carry out at least 20 mentored cases in practice after the course and our programme helps you accomplish this once you have sourced a mentor. We can help you find a mentor with our extensive list, including some of the team at UKSedation. Once at least 20 cases are completed mentored, you are then free to carry out IV Sedation independently. THE FEES WILL BE £1100 FOR DENTISTS AND £550 FOR DENTAL NURSES, INCLUDING ALL RESOURCE MATERIALS. IF BOOKING DENTIST AND NURSE TOGETHER USE PRODUCT CODE “UKSEDATION250” TO GET A £250 DISCOUNT ON THE NURSES TICKET. New course also delegates get access to exclusive offers from dental sedation equipment providers Once mentoring is completed, there is an additional fee of £150 for final certification. Key features of the course: Comprehensive Curriculum: Covering everything from patient assessment, sedation techniques, monitoring, and emergency protocols, our course gives you all the tools you need to safely administer dental sedation. Expert Instruction: Our instructors are experienced sedation practitioners who are passionate about sharing their knowledge. CPD Certified: The course is fully accredited for Continuing Professional Development, providing 14 verifiable sedation CPD hours upon completion. Post-course Support: We’re committed to your ongoing development, offering post-course support and resources to help you apply your new skills confidently in your practice. Mentoring – at UkSedation we can provide the mentors to visit your practice to help you with your required 20 cases (charged separately). We will train you in how to find the patients who would benefit from sedation and to have that discussion with them. Whether you’re a newly qualified dentist or nurse or a seasoned practitioner looking to expand your services, our New Starter Sedation Course is the perfect stepping stone to elevate your practice and help many more patients visit the dentist. Don’t miss out on this fantastic opportunity to enhance your professional skills, deliver better patient care, and grow your practice. Limited spaces are available, so sign up today and take the next step in your dental career with the UK Sedation & Mellow Dental’s New Starter Sedation Course. Course Includes 24 Lessons 14 Quizzes Course Certificate About Instructor Rob Endicott Roy Bennett Dr Roy Bennett is the director of Mellow Dental Training, IACSD accredited trainer and mentor and former director of Portmans Dental Care. Dr Rob Endicott is an IACSD accredited trainer and mentor and is the ViceChair of the ADAS (Association of Dental Anaesthetists and Sedationists)

Introduction to Exploration and Production for New Engineers and Non-Technical Professionals in Oil & Gas (2 Days)
By EnergyEdge - Training for a Sustainable Energy Future
Enhance your knowledge of exploration and production in oil & gas with EnergyEdge's course. Ideal for new engineers and non-technical professionals.

Introduction to Exploration and Production for New Engineers and Non-Technical Professionals in Oil & Gas (2 Days)
By EnergyEdge - Training for a Sustainable Energy Future
Enhance your knowledge of exploration and production in oil & gas with EnergyEdge's course. Ideal for new engineers and non-technical professionals.

Caring for People with Psychosis and Schizophrenia (non-RQF)
By Guardian Angels Training
Gain comprehensive understanding of caring for individuals with psychosis and schizophrenia. Enhance knowledge and skills to provide effective care and support. Designed for caregivers, family members, support workers, and healthcare professionals.

Recognising and Responding to Acutely Unwell Individuals
By Guardian Angels Training
Gain the knowledge and skills to identify acute illness in patients with our "Recognising and Responding to Acutely Unwell Individuals" course. Improve patient outcomes and prevent deterioration in various healthcare settings. Enroll now.

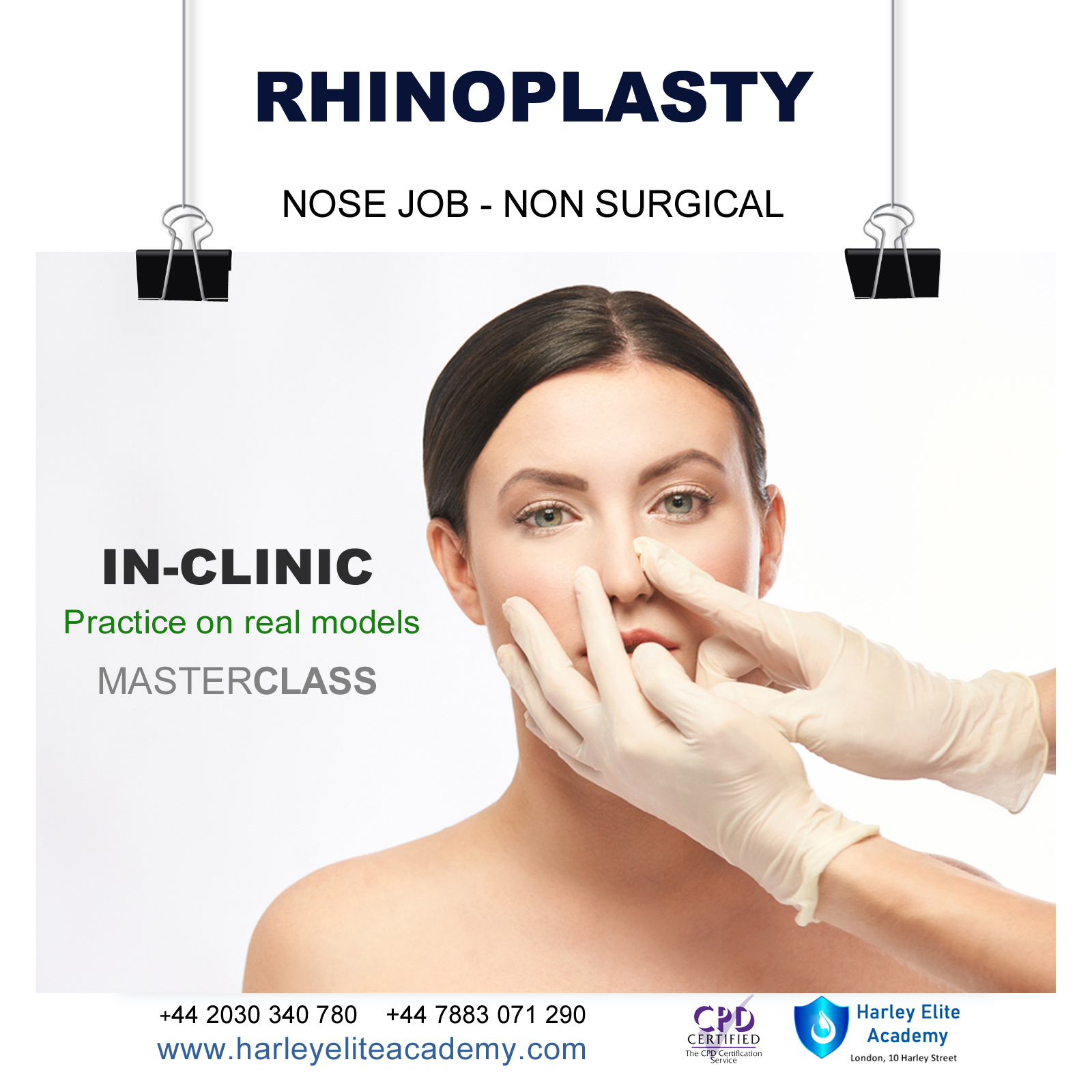
RHINOPLASTY NOSE JOB COURSE
By Harley Elite Academy (HeLa)
MASTERCLASS 8 CPD POINTS 1 DAY INTENSIVE COURSE ONLINE or IN-CLINIC NOTE! After booking we will contact you for scheduling the exact course date! Courses dates are subject to change due to mentors availability. We will inform you via email if a date becomes available! On this course, we aim to help you master a technique that will set you apart from most routine cosmetic treatment providers and enable you to step into the future of advanced cosmetic. Training THEORY will enable you to understand: Anatomy Vascular Supply, Nerves on the face Contraindications Patient consultation ONE-TO-ONE Training Nose Job Masterclass You will perform this procedure on live models under the supervision and guidance of highly experienced aesthetic practitioners. You will be trained under ENT specialist. We will give you all the knowledge you need for a safe technique in your practice. A certification of training will be provided upon completion of the course. You need to be medically qualified as a doctor, dentist, nurse, pharmacist or paramedic with full governing body registration and have completed a Foundation Filler Course and to have administered a number of cases. Additional information ATTENDANCE ONLINE (Theory), IN CLINIC (Practice) COURSE LEVEL EXPERT | Masterclass Course
Effective Negotiation Skills for Business Success (In-Person)
By IIL Europe Ltd
Effective Negotiation Skills for Business Success (In-Person) Become skillful at dealing with unworkable differences - situations where there appears to be no acceptable compromise or acceptable solution. This interactive workshop shows you how to work towards agreements where all parties are satisfied that they have reached a wise outcome efficiently, and where they can come back to the table in the future because the relationship is intact. You will have the opportunity to re-visit a difficult / challenging negotiation that you experienced in the past as well as take part in progressively more challenging case studies that are tailored to the work you do. You will enhance your personal and professional life with powerful new negotiating skills. The goal of this workshop is to improve your negotiation skills by helping you to identify your own preferred negotiation style and strategies, and to learn about the need to plan for any upcoming negotiation. The workshop is also designed for you to experience typical negotiation situations at certain key points of the life cycle of a project, enabling you to develop an awareness of your effectiveness during negotiations at these stages. The majority of time is spent on practicing newly presented negotiation techniques and receiving feedback on application for further development and improvement. What you will Learn At the end of this program, you will be able to: Identify your preferred negotiation style and strategies Examine the Principled Negotiation Model Adapt your negotiation strategy to the progress of the negotiation Experience typical negotiation situations during the life cycle of a project Develop an awareness of your effectiveness during negotiations Improve your negotiation skills Negotiation Model Negotiation defined Negotiation phases Common approaches to negotiating Principled Negotiating Principled negotiation and the four rules Best alternative to a negotiated agreement Project Negotiation Simulation: Part 1 and Part 2 Project Negotiation Simulation: Part 1 Project Negotiation Simulation: Part 2 Negotiating Cases Negotiating cases Summary and Next Steps

Search By Location
- Pha Courses in London
- Pha Courses in Birmingham
- Pha Courses in Glasgow
- Pha Courses in Liverpool
- Pha Courses in Bristol
- Pha Courses in Manchester
- Pha Courses in Sheffield
- Pha Courses in Leeds
- Pha Courses in Edinburgh
- Pha Courses in Leicester
- Pha Courses in Coventry
- Pha Courses in Bradford
- Pha Courses in Cardiff
- Pha Courses in Belfast
- Pha Courses in Nottingham