- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
4418 Pha courses
Effective Negotiation Skills for Business Success: In-House Training
By IIL Europe Ltd
Effective Negotiation Skills for Business Success: In-House Training Become skillful at dealing with unworkable differences - situations where there appears to be no acceptable compromise or acceptable solution. This interactive workshop shows you how to work towards agreements where all parties are satisfied that they have reached a wise outcome efficiently, and where they can come back to the table in the future because the relationship is intact. You will have the opportunity to re-visit a difficult / challenging negotiation that you experienced in the past as well as take part in progressively more challenging case studies that are tailored to the work you do. You will enhance your personal and professional life with powerful new negotiating skills. The goal of this workshop is to improve your negotiation skills by helping you to identify your own preferred negotiation style and strategies, and to learn about the need to plan for any upcoming negotiation. The workshop is also designed for you to experience typical negotiation situations at certain key points of the life cycle of a project, enabling you to develop an awareness of your effectiveness during negotiations at these stages. The majority of time is spent on practicing newly presented negotiation techniques and receiving feedback on application for further development and improvement. What you will Learn At the end of this program, you will be able to: Identify your preferred negotiation style and strategies Examine the Principled Negotiation Model Adapt your negotiation strategy to the progress of the negotiation Experience typical negotiation situations during the life cycle of a project Develop an awareness of your effectiveness during negotiations Improve your negotiation skills Negotiation Model Negotiation defined Negotiation phases Common approaches to negotiating Principled Negotiating Principled negotiation and the four rules Best alternative to a negotiated agreement Project Negotiation Simulation: Part 1 and Part 2 Project Negotiation Simulation: Part 1 Project Negotiation Simulation: Part 2 Negotiating Cases Negotiating cases

IASSC Lean Six Sigma Black Belt (Exam Included) – 8 Day Evening Live Online Class
By Hudson
8 day live online class Total duration: 24 hours Classes run once a month from 18:30-21:30 GMT (13:30-14:30 EST) over an 8 day period

PV03: Drug Safety and Pharmacovigilance
By Zenosis
Drug safety monitoring and risk management are vitally important for medicinal product developers, licence holders and clinical investigators. In addition to their duty to protect public health, increasingly tight regulation and potentially massive payments to litigants provide strong incentives for pharmaceutical and biotechnology companies to ensure that they maintain efficient systems for drug safety / pharmacovigilance and that all staff are aware of the basic requirements. This course will provide them with an overview of the most important aspects of this discipline, both before and after marketing of products, especially as they apply in Europe and the USA.

Certified Associate in Project Management (CAPM) Exam Prep: Virtual In-House Training
By IIL Europe Ltd
Certified Associate in Project Management (CAPM)® Exam Prep: Virtual In-House Training This course gives you the knowledge you need to pass the exam and covers CAPM®-critical information on project management theory, principles, techniques, and methods Are you planning on taking the CAPM® examination? This course gives you the knowledge you need to pass the exam and covers CAPM®-critical information on project management theory, principles, techniques, and methods. You'll also have an opportunity for practical applications and time to review the kinds of questions you'll find in the CAPM® Exam. What you Will Learn Apply for the CAPM® Examination Develop a personal exam preparation plan Describe the structure, intent, and framework principles of the current edition of the PMBOK® Guide Explain the PMBOK® Guide Knowledge Areas, as well as their inter-relationships with the each other and the Process Groups Getting Started Program orientation The CAPM® certification process Certified Associate in Project Management (CAPM®) Examination Content Outline CAPM® eligibility requirements Code of Ethics and Professional Conduct Application options Foundation Concepts Skills and qualities of a project manager Project management terminology and definitions Relationship of project, program, portfolio, and operations management Project lifecycle approaches Project Integration Management Review Project Integration Management Knowledge Area Develop Project Charter Develop Project Management Plan Direct and Manage Project Work Manage Project Knowledge Monitoring and Controlling Perform Integrated Change Control Close Project or Phase Project Stakeholder Management Review Project Stakeholder Management Knowledge Area Identify Stakeholders Plan Stakeholder Engagement Manage Stakeholder Engagement Monitor Stakeholder Engagement Project Scope Management Review Project Scope Management Knowledge Area Plan Scope Management Collect Requirements Define Scope Create WBS Validate Scope Control Scope Project Schedule Management Review Project Schedule Management Knowledge Area Plan Schedule Management Define Activities Sequence Activities Estimate Activity Durations Develop Schedule Control Schedule Project Cost Management Review Project Cost Management Knowledge Area Plan Cost Management Estimate Costs Determine Budget Control Schedule Project Resource Management Review Project Resource Management Knowledge Area Plan Resource Management Estimate Activity Resources Acquire Resources Develop Team Manage Team Control Resources Project Quality Management Review Project Quality Management Knowledge Area Plan Quality Management Manage Quality Control Quality Project Risk Management Review Project Risk Management Knowledge Area Plan Risk Management Identify Risks Perform Qualitative Risk Analysis Perform Quantitative Risk Analysis Plan Risk Responses Implement Risk Responses Monitor Risks Project Communications Management Review Project Communications Management Knowledge Area Plan Communications Management Manage Communications Monitor Communications Project Procurement Management Review Project Procurement Management Knowledge Area Plan Procurement Management Conduct Procurements Control Procurements Summary and Next Steps Program Review Mock CAPM® Exam Getting Prepared for the CAPM® Exam After the CAPM® Exam

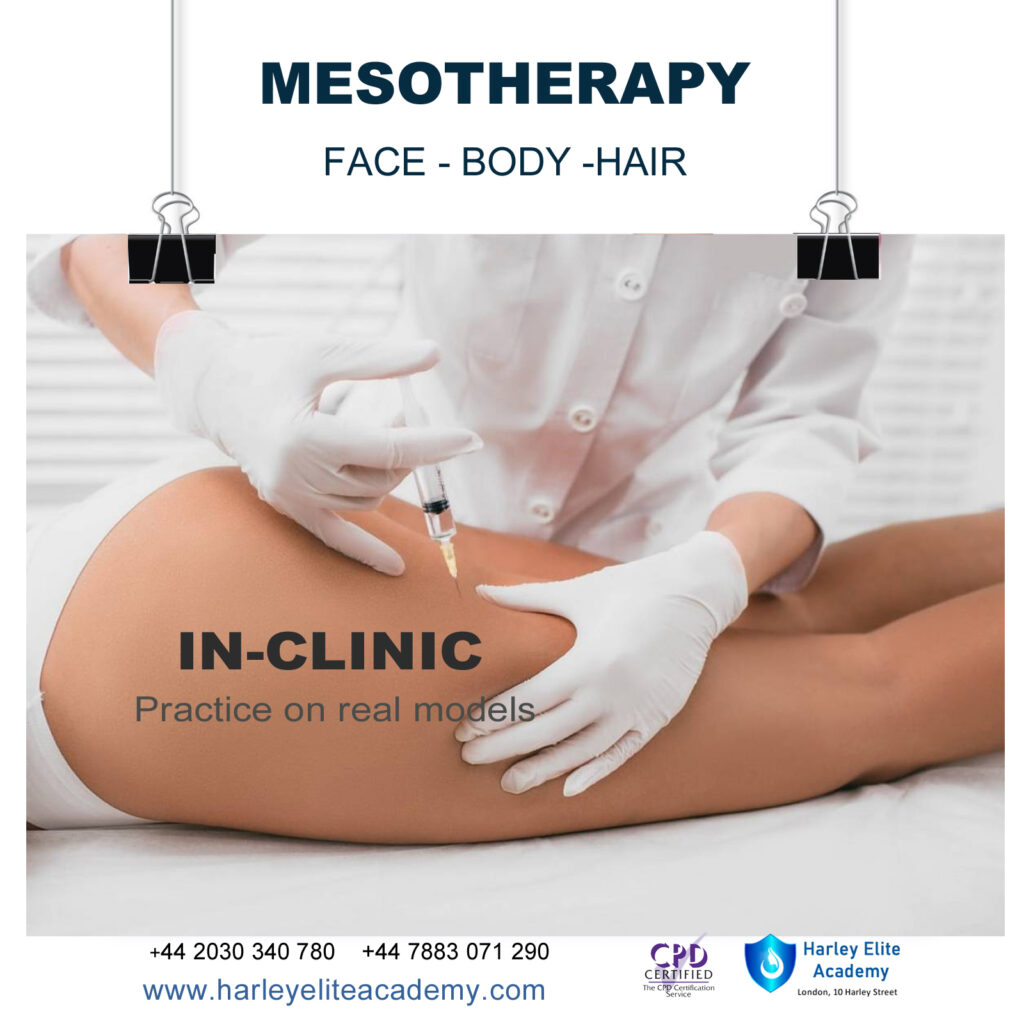
MESOTHERAPY, FACE, BODY, HAIR COURSE
By Harley Elite Academy (HeLa)
MASTERCLASS 8 CPD POINTS 1 DAY INTENSIVE COURSE ONLINE or IN-CLINIC NOTE! After booking we will contact you for scheduling the exact course date! Courses dates are subject to change due to mentors availability. We will inform you via email if a date becomes available! Nose Job Masterclass You will perform this procedure on live models under the supervision and guidance of highly experienced aesthetic practitioners. You will be trained under ENT specialist. We will give you all the knowledge you need for a safe technique in your practice. A certification of training will be provided upon completion of the course. Practice will enable you to learn in 1-day ONE-TO-ONE Training You will also have the opportunity to view a mesotherapy treatment demonstration at the conclusion of the training. Upon successful completion of the course, you will receive a certificate and title of Mesotherapy Certified Practitioner. You need to be medically qualified as a doctor, dentist, nurse, pharmacist or paramedic with full governing body registration and have completed a Foundation Filler Course and to have administered a number of cases. Additional information ATTENDANCE ONLINE (Theory), IN CLINIC (Practice) COURSE LEVEL EXPERT | Masterclass Course
Implementing Good Clinical Laboratory Practice
By Research Quality Association
Course Information Join our comprehensive course, meticulously designed to equip individuals implementing Good Clinical Laboratory Practice (GCLP) within laboratories handling samples from clinical trials. Delve into the current regulatory landscape governing laboratory work supporting clinical trials, referencing key guidelines such as the ICH Guideline for Good Clinical Practice, the Clinical EU Trials Directive, relevant regulations, and leveraging insights from the RQA guidance document on GCLP. Is this course for you? This course is tailored for laboratory managers, analysts, investigators, trial coordinators, monitors, and auditors operating in diverse settings such as pharmaceutical company laboratories, central laboratories, contract research organisations, hospital laboratories, clinics, and investigator sites. This course will give you: Guidance on effectively interpreting and applying GCLP within the broader framework of Good Clinical Practice (GCP) Insight into the seamless integration of GCLP within clinical programmes (GCP) Practical strategies for implementing GCLP in the nuanced environment of clinical research laboratories The chance to update your knowledge with the latest interpretations and guidance on clinical laboratories by the UK Medicines and Healthcare Products Regulatory Agency (MHRA) Access to a seasoned panel of speakers with extensive expertise A unique opportunity to deepen your understanding of GCLP's application across diverse scenarios. Engage in: Lively discussions to foster ideas Problem-solving sessions targeting specific challenges Detailed exploration of specific aspects within the realms of GCP and GCLP. Tutors Tutors will be comprised of (click the photos for biographies): Vanessa Grant -, - Louise Handy Director, Handy Consulting Ltd Tim Stiles Consultant, Qualogy Ltd Programme Please note timings may be subject to alteration. Day 1 08:50 Registration 09:00 Welcome and Introduction 09:20 Good Clinical Practice and the Requirements of Good Clinical Laboratory Practice A review of Good Clinical Practice and its requirements for the laboratory analysis of samples from a trial. The thought processes behind the development of Good Clinical Laboratory Practice, its objective, scope, interpretation and application are explained. 10:00 Safety and Ethical Consideration Informed consent, confidentiality, expedited reporting, blinding and unblinding and serious breaches of the GCP are discussed. 10:40 Break 10:55 Organisation and Personnel Responsibilities within GCP and the Laboratory The responsibilities of key functions that should exist within a clinical laboratory including personnel records of training and competence are discussed. 11:30 Staff Training and Training Records Personnel records of training and competency assessments are discussed. 11:45 Laboratory Facilities, Equipment and Materials Suitable facility design, organisation and operation will be discussed. The calibration, validation and maintenance of equipment used in the conduct of sample analysis are examined, as are the suitability of materials and the identification and labelling of reagents and solutions. 12:30 Lunch 13:15 Workshop 1 - Facilities, Equipment and Responsibilities Some practical problems with regard to the facilities, equipment and responsibilities are explored. 13:45 Workshop 1 - Feedback 14:15 Computer Systems Validation Systems, including computerised systems, used in the analysis, collection and reporting of results should be appropriately tested, operated and controlled. What this means in practice is discussed. 14:45 Trial Protocols, Analytical Plans During this session we examine the purpose, content, control and change of these important documents. 15:30 Break 15:45 Workshop 2 - SOPs, Clinical Protocols, Analytical Plans and Validation The practicalities of managing and documenting the planning phase of analytical work on a trial are explored along with computerised system validation. 16:30 Workshop 2 - Feedback 17:00 Close of Day Day 2 09:00 Conduct of the Work and Quality Control Many of the issues that surround the conduct of sample collection, shipment, storage, analysis and management of Analytical Methods are discussed. This includes the quality control of the assay that may be employed and Quality Control checks. 10:00 Deviation Management The expectations around deviations and CAPA are discussed. 10:15 Workshop 3 - Conduct of the Work and Quality Control Practical work conduct and quality control issues are explored. 10:45 Break 11:00 Workshop 3 - Feedback 11:30 Source Data, Data Integrity, Records and Reports The creation and subsequent management of source data and records, data integrity, are discussed, together with the process of reporting analytical results. 12:10 Workshop 4 - Data, Records and Reports Practical problems with data, records and reports are investigated. 12:45 Lunch 13:30 Workshop 4 - Feedback 14:00 Quality Audit The requirements for and purpose of quality audits are discussed. The difference between quality audit and quality control are explained along with the role of the quality audit staff and their interaction with the analytical project managers, laboratory management and study staff. 14:40 Risk Management How should we assess risk and how can we use the process to assist in evaluation of audit findings. 15:15 Break 15:30 Regulatory Inspection The conduct of regulatory inspections and current expectations of the inspectors. Preparation for inspections and conduct during them will be discussed. 16:00 Panel Session This panel session will address any outstanding issues raised by the delegates. 16:15 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Develop

Healthcare Hero Instructor Training Certification: Level 1
By HFX Training
At HFX Training, we are passionate about business simulations because they bring learning to life through hands-on experience. This Level 1 Certification Program is the first step in our Instructor Training Certification Pathway, equipping you to confidently use the Healthcare Hero simulation in your teaching environment. In this program, you will experience the simulation as a student, navigating its complexities and dynamics. Upon successfully completing Level 1, you’ll be ready to advance to Level 2 Training, where you will deepen your expertise in simulation facilitation and administration. The program is cohort-based, and the timetable is coordinated in advance through discussions with the HFX team to ensure maximum participation. For more information on the program structure, instructional materials, and certification requirements, please read on. What to Expect: The Healthcare Hero simulation places you in charge of a fictional hospital. Over eight simulation rounds - each representing one quarter of business operations - you will make strategic decisions to optimize patient care while managing staffing, capacity, quality, and finances. Throughout the simulation, you’ll navigate challenges like healthcare and economic crises, all while balancing the needs of multiple stakeholders. Your ultimate goal is to outperform competing hospitals managed by other participants in the program. Program and Certification Requirements This program equips you to confidently lead the simulation by requiring more than attendance or participation. To earn certification, you must: Thoroughly Prepare: Review all instructional materials and the scenario manual before the course begins. Fully Participate: Attend all Zoom sessions and complete each simulation round with focus and effort. Demonstrate Mastery: Show a comprehensive understanding of the simulation’s dynamics, decision-making processes, and strategies during practice and live rounds. Engage in Reflection: Actively participate in debrief sessions, assess your performance, and identify strategies for improvement. This certification ensures you are equipped to facilitate this complex simulation with confidence. Auditing the course is an option, but participants who choose to audit will not receive certification. Program Format The program is delivered intensively over one or more Zoom sessions, totaling six hours. This compressed format demands your full attention and focus to successfully navigate the simulation’s complexities. Eligibility Requirements: By registering for this training program, you acknowledge the following: You have discussed your needs with the HFX team and confirmed that the simulation aligns with your class or program objectives. You are an instructor at an academic institution. You agree to the confidentiality terms and commit not to distribute, disclose, or replicate any program content for purposes outside of this training. This program is offered in goodwill to academic instructors as a resource to enhance their teaching. As part of this agreement, you understand that all materials, including the simulation itself, are proprietary to HFX and its partners. Any use of these materials for purposes beyond this program, such as developing similar tools or simulations, is strictly prohibited. Course Structure: After registering, you will gain immediate access to the simulation’s instructional materials. This includes: The Scenario Manual: Your guide to understanding the game. The Capacity Planner Worksheet and Financial Planning Worksheet: Tools to help you strategize. The Strategy Planning Exercise: Designed to align your decisions with overall objectives. The Portal Guide: A walkthrough of the simulation platform. The program unfolds in four key phases: Phase 1: Pre Zoom Call / Study / Set Up Register using the button at the bottom of this page. Review the instructional materials thoroughly. Once the cohort is finalized, you will be sent your login credentials for the simulation itself. Try to submit decisions for the first practice round using the simulation portal at www.simulationportal.com. Phase 2: Play We run two practice rounds over the first 2 hours of the zoom call. This allows you to familiarize yourself with the platform and decision-making process, and think through what your strategy might be in the live game. The simulation resets ("zeroed") after the practice rounds. A brief session will clarify lessons learned and expectations for the live rounds. Over eight rounds, you’ll compete against other participants while refining your strategic decisions. Phase 3: Feedback Reflect on your performance during a structured debrief, including: Strengths and areas for improvement. Insights into competitors’ strategies. Strategic lessons learned, and applicability to local healthcare organizations. Phase 4: Implementation Planning Work with the HFX team to plan your first application of the simulation in a teaching environment. Certification Outcome Upon completing this program, you will earn the HFX Instructor Training Certification: Level One, signifying your expertise in conducting impactful simulation-based training using Healthcare Hero.

Các Mẫu Giày Tây Nam Cao Cấp Phù Hợp Cho Môi Trường Công Sở
By Các Mẫu Giày Tây Nam Cao Cấp Phù Hợp Cho Môi Trường Công Sở
Các Mẫu Giày Tây Nam Cao Cấp Phù Hợp Cho Môi Trường Công Sở Trong môi trường công sở, trang phục không chỉ thể hiện sự chuyên nghiệp mà còn tạo ấn tượng đầu tiên mạnh mẽ với đối tác và đồng nghiệp. Một phần quan trọng không thể thiếu của bộ trang phục công sở chính là giày tây. Giày tây nam không chỉ giúp bạn hoàn thiện vẻ ngoài lịch lãm mà còn phản ánh phong cách và sự tôn trọng quy tắc thời trang. Hôm nay, chúng ta sẽ cùng khám phá các mẫu giày tây nam Timan cao cấp phù hợp nhất cho môi trường công sở. 1. Giày Derby – Sự Pha Trộn Giữa Cổ Điển Và Hiện Đại Một trong các mẫu giày tây nam được ưa chuộng trong giới công sở là giày Derby. Với thiết kế mở dây, giày Derby mang đến sự thoải mái và dễ dàng trong việc điều chỉnh kích cỡ, phù hợp với những người có mu bàn chân cao. Giày Derby không chỉ thích hợp cho những dịp trang trọng mà còn dễ dàng kết hợp với các trang phục như vest, suit hay thậm chí là quần âu. Đặc điểm của giày Derby nằm ở sự đa dụng và thanh lịch. Bạn có thể chọn giày Derby da trơn cho những ngày làm việc quan trọng hoặc giày Derby da lộn cho phong cách công sở nhẹ nhàng và thoải mái hơn. Màu sắc phổ biến nhất cho giày Derby là đen và nâu, hai gam màu kinh điển mà bất kỳ quý ông nào cũng nên có trong tủ giày. 2. Giày Oxford – Biểu Tượng Của Sự Lịch Lãm Giày Oxford Nam GT80 Giày Oxford luôn nằm trong top đầu các mẫu giày tây nam dành cho môi trường công sở. Với thiết kế dây buộc kín, giày Oxford mang đến vẻ ngoài lịch lãm, sang trọng và rất phù hợp cho các dịp trang trọng như cuộc họp, thuyết trình, hay gặp gỡ đối tác. Điểm khác biệt của giày Oxford so với Derby nằm ở phần dây giày được buộc chặt vào phần thân trên, tạo nên sự gọn gàng và thanh lịch cho đôi chân. Giày Oxford thường được làm từ da cao cấp, tạo nên sự bền bỉ và độ bóng loáng sau thời gian sử dụng. Màu đen là sự lựa chọn phổ biến nhất cho giày Oxford vì tính thanh lịch và dễ kết hợp với trang phục công sở. Ngoài ra, những đôi giày Oxford màu nâu cũng là lựa chọn lý tưởng cho những ai muốn thể hiện phong cách thời trang hiện đại. 3. Giày Loafer – Sự Tiện Lợi Và Thoải Mái Trong số các mẫu giày tây nam cho môi trường công sở, giày Loafer nổi bật với sự tiện lợi và thoải mái. Không cần buộc dây, giày Loafer dễ dàng xỏ vào và tháo ra, giúp tiết kiệm thời gian nhưng vẫn giữ được vẻ ngoài lịch lãm. Giày Loafer được làm từ nhiều chất liệu khác nhau như da bóng, da lộn, hoặc vải cao cấp, mang lại sự đa dạng cho phong cách công sở. Giày Loafer thường được sử dụng trong những môi trường công sở thoải mái hơn, không quá đòi hỏi tính trang trọng. Bạn có thể phối giày Loafer với quần âu, áo sơ mi hoặc cả vest để tạo nên một phong cách công sở đầy phóng khoáng nhưng vẫn chuyên nghiệp. 4. Giày Monk Strap – Phong Cách Sang Trọng Và Độc Đáo Giày Nam Monk Strap GT77 Monk Strap là một trong các mẫu giày tây nam độc đáo, mang lại phong cách riêng biệt cho người mang. Thay vì sử dụng dây buộc truyền thống, giày Monk Strap được thiết kế với khóa cài, tạo nên điểm nhấn sang trọng và khác biệt. Mẫu giày này phù hợp cho những quý ông yêu thích sự phá cách nhưng vẫn muốn giữ được tính trang trọng khi đến văn phòng. Giày Monk Strap có nhiều loại, từ kiểu khóa đơn đến khóa kép. Những đôi giày này thường được làm từ da cao cấp, mang lại độ bền cao và phong cách thời thượng. Màu sắc phổ biến của Monk Strap là đen, nâu và đôi khi là màu burgundy cho những ai muốn tạo nên điểm nhấn trong trang phục. ➔➔➔ Tham khảo những mẫu giày tây nam đẹp, thời thượng được săn đón năm nay: https://plo.vn/nhung-mau-giay-tay-nam-dep-thoi-thuong-duoc-san-don-nam-nay-post754395.html 5. Giày Brogue – Sự Phá Cách Tinh Tế Cuối cùng trong danh sách các mẫu giày tây nam cao cấp cho môi trường công sở là giày Brogue. Đặc điểm nhận diện của giày Brogue chính là những lỗ đục tinh tế trên bề mặt da, tạo nên vẻ ngoài khác biệt và cuốn hút. Giày Brogue thường được lựa chọn bởi những quý ông yêu thích sự sáng tạo trong phong cách, nhưng vẫn muốn giữ sự chuyên nghiệp. Giày Brogue có thể kết hợp với nhiều kiểu trang phục công sở khác nhau, từ quần âu, vest cho đến áo sơ mi. Tuy nhiên, giày Brogue sẽ phù hợp hơn với những dịp công sở không quá trang trọng, nơi bạn có thể thoải mái thể hiện cá tính qua trang phục. Cách Lựa Chọn Giày Tây Nam Cao Cấp Cho Môi Trường Công Sở Giày Oxford Nam GT82 Khi chọn các mẫu giày tây nam cho môi trường công sở, bạn cần lưu ý đến các yếu tố sau để đảm bảo sự phù hợp và thoải mái: Chất liệu: Chất liệu da là lựa chọn hàng đầu khi mua giày tây nam cho công sở. Da bò, da lộn, hoặc da bóng đều mang lại vẻ ngoài sang trọng, bền đẹp theo thời gian. Màu sắc: Màu đen và nâu là hai màu phổ biến nhất cho giày tây nam trong môi trường công sở. Chúng không chỉ dễ phối đồ mà còn tạo nên sự chuyên nghiệp và lịch lãm. Thiết kế: Tùy thuộc vào tính chất công việc, bạn có thể chọn giày buộc dây như Oxford, Derby hoặc giày không dây như Loafer, Monk Strap để phù hợp với phong cách cá nhân và quy định của công ty. Độ thoải mái: Dù mẫu giày có đẹp đến đâu, sự thoải mái vẫn là yếu tố quan trọng nhất. Hãy chọn những đôi giày có độ ôm chân vừa phải, đảm bảo bạn có thể di chuyển cả ngày mà không bị đau chân. Kết Luận Môi trường công sở đòi hỏi sự chuyên nghiệp và chỉn chu trong cách ăn mặc, và giày tây nam là một phần quan trọng trong việc hoàn thiện phong cách của bạn. Dù bạn chọn giày Oxford thanh lịch, Derby thoải mái hay Monk Strap độc đáo, hãy luôn đảm bảo rằng đôi giày không chỉ phù hợp với phong cách cá nhân mà còn mang lại sự thoải mái cho cả ngày dài làm việc. Hy vọng qua bài viết này, bạn đã có thêm nhiều sự lựa chọn trong số các mẫu giày tây nam cao cấp phù hợp cho môi trường công sở, giúp bạn tự tin hơn trong mỗi bước chân.

Brazilian Buttock Lift ONLINE MASTERCLASS (BBL)
4.9(74)By Harley Elite Academy | Cosmetic Medicine Courses
This course is intended for delegates who are now doing dermal fillers or whom are advanced injectors. The Brazilian Butt Lift training includes improving shape, volumizing and sculpting the area with dermal filler. We use Hyacorp MFL 2 or Genefill Products MASTERCLASS GROUP 8 CPD POINTS 1 DAY COURSE ONLINE During the Online Training day you will learn the following: Anatomy and Physiology Consultation for patients Consent forms Treatment information After care Dezinfection & Safety Indications and Contra-indications Risks, side effects Dealing with complications Marketing tips Certificate upon completion CPD All under the supervision of our fully trained aesthetics practitioners, with Medical background . You will practice on real models and optional extra Mentoring date can be provided if required! BBL is the newest of the body augmentation treatments in the cosmetic/aesthetic industry Masterclass. ONLINE BBL TRAINING COURSE IN LONDON Practice in 1-day Course Group Training Non-Surgical Liquid Brazilian Butt Lift with Hyaluronic Acid ( difference Fillers )Hayacorp MFL 2 ,Genefill contour using cannulas. Brazilian Butt lift with Fillers also called Liquid BBL has become one of the most popular non-surgical cosmetic procedures. Client’s are more than ever looking for non-surgical alternatives to the most popular cosmetic procedures. Take advantage of this ever-growing market with our one-day intensive course, extend you level of injectable procedure. Our Buttock Augmentation training course you will learn how to safely administrate the HA and effectively carry out these procedures to help your clients achieve a firmer, rounder buttock whilst saving your clients thousands on undergoing invasive surgery. The Brazilian Buttock Lift, training course consists in learning Master class injection techniques and application for the most frequently requested areas of the body under professional supervise with some of the best experts of Academy on central London. You can extend the training with Legs, Arms and Calves. With Harley Elite Academy you can learn to practice in cosmetic medicine what the most celebrities want! We will cover pertinent information including mechanism of action technique of safety, dealing with complications, dilution guidelines, and more., providing injectable protocol for the products. Contraindications Complications Management Post treatment advice You need to be medically qualified as a doctor, dentist, nurse, pharmacist or paramedic with full governing body registration and have completed a Foundation Filler Course and to have administered a number of cases.

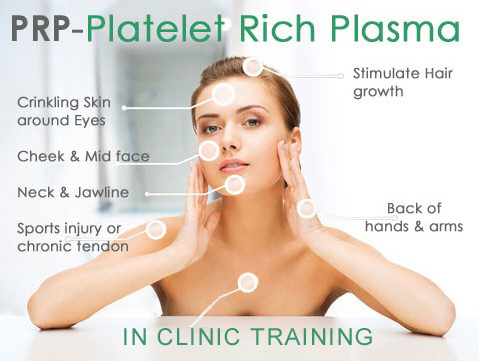
Platelet Rich Plasma Skin & Hair | JOIN IN-CLINIC CLASS
4.9(74)By Harley Elite Academy | Cosmetic Medicine Courses
CLINICAL PRP Sports medicine Traumatology Ophthalmic Burn trauma Wound healing –diabetic foot Skin grafting Dentistry-sinus lift Tooth implants. PRP theory & equipment: Training Online Theory will enable you to understand: Anatomy Vascular Supply, Contraindications Patient consultation Complications Management Post treatment advice Dealing with equipment | Suppliers A certification of training will be provided upon completion of the course. Aesthetic PRP Skin rejuvenation Hair restoration Fat grafting in combination PRP Post laser Acne & Rosacea Acne scar Tissue Volumisation alternative of HA fillers Aesthetic gynaecology /urology. Plathelet Rich Plasma We will cover pertinent information including mechanism of action, safety and efficacy issues, management and treatment of complications, dilution guidelines, and more. Hands on practical session – skin rejuvenation and hair loss Extraction, Preparation and Dosage Management Injection techniques – face, neck and head (hair loss); also the use of cannula Upon successful completion of the course, you will receive a certificate and title of PRP Certified Practitioner. MASTER CLASS PRP & PRF During the course we are providing . Taking blood and how to use a Centrifuge . PRP injecting techniques in face neck and décolletage hands. PRP Microneedling using a DERMAPEN. Combination treatment PRP with Mesotherapy. MECHANISM OF ACTION Platelets + Leucocytes form 3D mesh release of GF Chemo attraction and migration of macrophages and stem cells Stem cells proliferates by mitosis Stem cells undergo differentiation process BENEFIT FROM PRP TREATMENT & THERAPYExperience the advantages of PRP treatment and therapy, utilizing autologous blood with natural growth factors for disease-free and hypoallergenic benefits. Boost wound healing by regulating mitosis, proliferation, and differentiation, enhancing tissue with collagen, elastin, and hyaluronic acid. Benefit from improved tissue oxygenation, nutrition flow, and support for procedures like hair transplants, fat transfers, and skin grafts.PRP works effectively in skin rejuvenation, facial resurfacing, microneedling, and combines well with HA, PDO threads, skin boosters, peeling, or CO2 lasers. It also proves beneficial for hair restoration, showing positive results in various protocols for Androgenic alopecia and age-related hair loss.PRP where works .Skin rejuvenation-facial resurfacing.application-injection alone. Microneedling Combination with HA,Combination with PDO threads,Skin boosters , peeling or CO2 lasers Hair restoration, Multiple protocols with positive results Evidence for improvement of: Androgenic alopecia-male and females, “spot hair lost” Improvement of age related hair loss. You need to be medically qualified as a doctor, dentist, nurse, pharmacist or paramedic with full governing body registration and have completed a Foundation Filler Course and to have administered a number of cases. MASTERCLASS 8 CPD POINTS 1 DAY INTENSIVE COURSE HANDS-ON REAL MODELS
Search By Location
- Pha Courses in London
- Pha Courses in Birmingham
- Pha Courses in Glasgow
- Pha Courses in Liverpool
- Pha Courses in Bristol
- Pha Courses in Manchester
- Pha Courses in Sheffield
- Pha Courses in Leeds
- Pha Courses in Edinburgh
- Pha Courses in Leicester
- Pha Courses in Coventry
- Pha Courses in Bradford
- Pha Courses in Cardiff
- Pha Courses in Belfast
- Pha Courses in Nottingham