- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
Good Laboratory Practice for Study Directors, Principal Investigators, Study Staff and Management
By Research Quality Association
Course Information Embark on our GLP course offering extensive guidance and pragmatic support tailored for individuals serving as Study Directors or Principal Investigators overseeing non-clinical safety studies on pharmaceuticals, agricultural, and industrial chemicals within the realm of Good Laboratory Practice (GLP). This comprehensive programme extends its benefits to study staff and management operating in GLP-compliant environments. The course extensively covers the current OECD GLP Principles and UK GLP legislation, while also referencing international standards, regulations, and guidelines pertinent to the field. Benefits of this course: Practical help and guidance on the interpretation and application of GLP An opportunity to update your knowledge of GLP with the current interpretation of requirements Access to an experienced panel of speakers Information on how other organisations address GLP issues An opportunity to improve your understanding of the GLP requirements as they are applied in different situations. This course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Examine particular aspects of GLP Learn from the experience of others. Tutors Tutors will be comprised of (click the photos for biographies): Tim Stiles Consultant, Qualogy Ltd Tony Woodall Head of Quality Assurance, Alderley Analytical Gill Armour Study Monitor Team Leader, AstraZeneca Jane Elliston Senior Quality Assurance Auditor, Battelle UK Vanessa Grant -, - Jeanet Logsted CEO, Scantox Programme Please note timings may be subject to alteration. Day 1 09:00 Registration 09:15 Welcome and Introductions 09:35 Development of Good Laboratory Practice A review of the history of GLP, its current scope and application, with a synopsis of current European and international standards. 10:05 Roles and Responsibilities The responsibilities of study director, test facility, management and study staff in the conduct of a GLP study. 10:45 Break 11:00 The Roles and Responsibilities of the Study Director and Test Facility Management The role of the study director in the management and control of a study, as defined by GLP, and management's roles are explored. 11:45 Multi-site Studies What is a multi-site study and when should such concepts be applied on a study. The role of the study director and principal investigator in the planning, conduct and reporting of multi-site study are explored. 12:30 Study Plan (Protocols) GLP requirements for the preparation of a study plan, content, authorisation, amendments and deviations are discussed. 13:00 Lunch 13:45 Workshop 1 - The Study Plan Some practical problems with study plans and amendments explored. 14:45 Workshop 1 - Feedback 15:00 Standard Operating Procedures The control, content and authorisation of SOPs and the principles behind the practice. 15:30 Break 15:45 Workshop 2 - Practical Study Conduct Problems Dealing with practical problems encountered during the conduct of studies. 16:40 Workshop 2 - Feedback 17:15 Close of Day Day 2 09:00 Questions and Answers Discussion of issues raised by course delegates. 09:20 Quality Assurance The interactions between QA, management, study director and principal Investigator are discussed as is QAs role when conducting a multi-site study. 10:00 The Final Report The content of the final report and the role of those involved in its preparation and approval. Specific reporting requirements when conducting a multi-site study are also explained. 10:30 Break 10:45 Workshop 3 - Final Report Problems Practical problems of report preparation including compliance statements. 11:30 Workshop 3 - Feedback 12:00 Management of Raw Data and Records A view on how records and materials are managed and archived in compliance with GLP. 12:45 Lunch 13:30 Workshop 4 - Data and Sample Management Issues Dealing with data and sample management issues. 14:15 Workshop 4 - Feedback 14:45 Regulatory Inspection Government monitoring for compliance with Good Laboratory Practice. 15:15 Panel Session This panel session will address any outstanding issues raised by delegates. 15:45 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Develop

An Understanding of Nasogastric Tube (NGT) Insertion and Feeding Training
By Guardian Angels Training
Nasogastric tube training ensures healthcare professionals have the correct clinical knowledge and skills to provide excellent care and support for individuals who may require treatment via NG tube for nutrition, hydration or medication.

An Understanding of Physiological Observations
By Guardian Angels Training
Enhance patient care with our "Understanding Physiological Observations" course. Gain comprehensive knowledge and skills to accurately assess and interpret vital signs and other measurements.
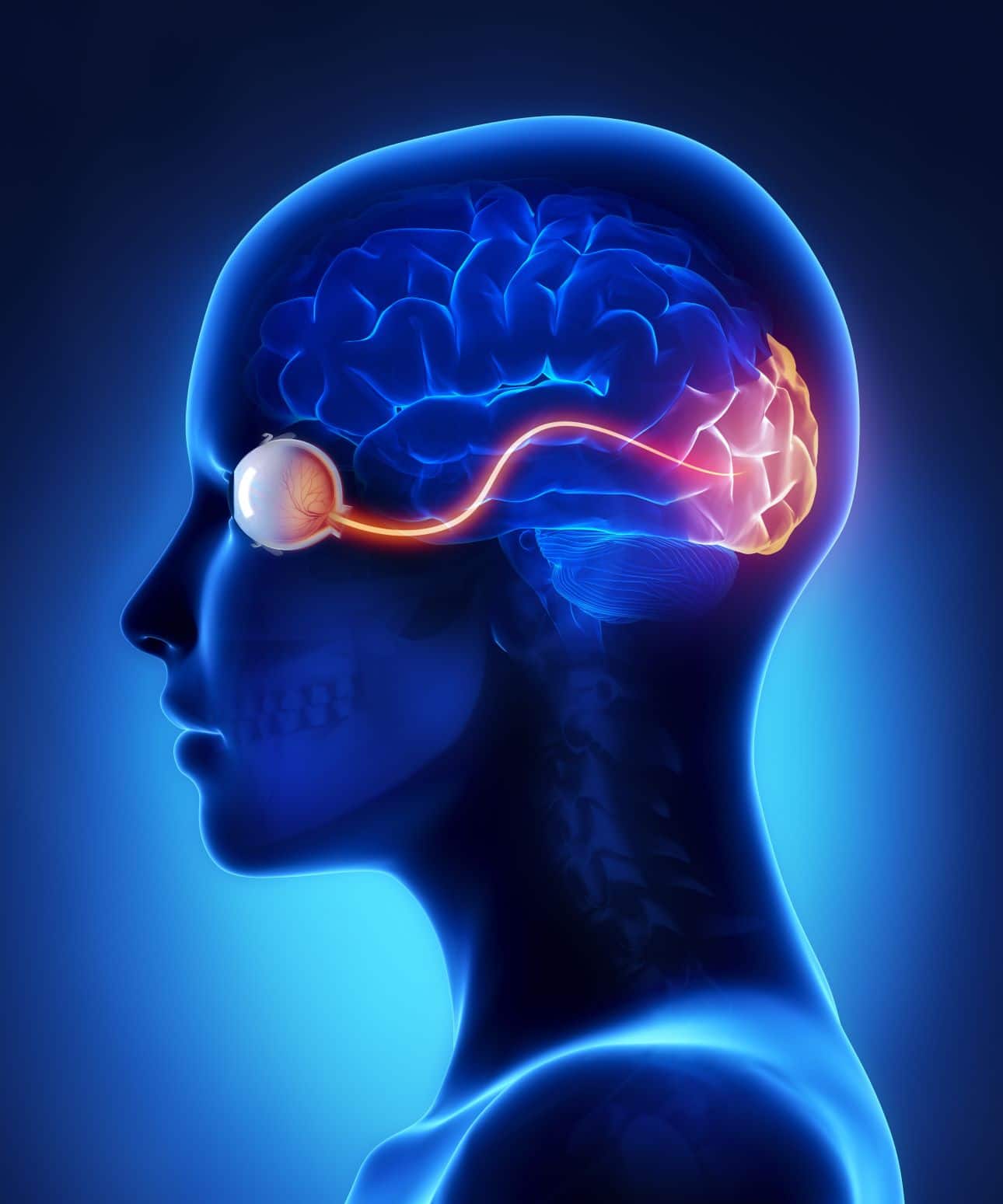
An Understanding of Ear Assessment and Care (including Ear Irrigation)
By Guardian Angels Training
Enhance your ear assessment and care skills with our comprehensive course on ear irrigation. Learn anatomy, assessment techniques, and safe practices for optimal ear health.
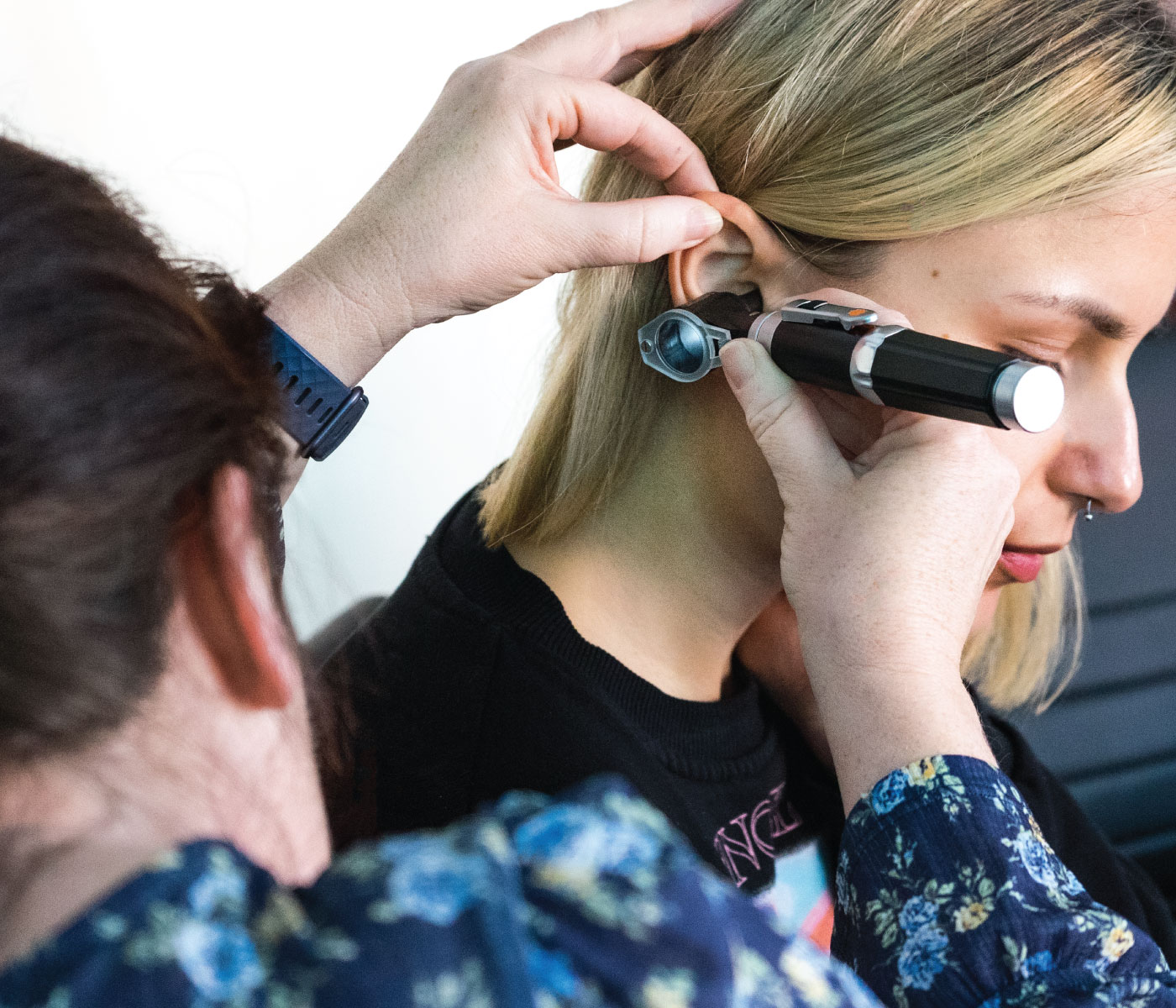
Buccal (Oromucosal) Midazolam Competent Reviewer / Assessor
By Guardian Angels Training
The Buccal (Oromucosal) Midazolam Competent Reviewer / Assessor Training is designed to provide participants with comprehensive knowledge and skills necessary to assess and review the administration of buccal (oromucosal) midazolam for the management of acute prolonged and repetitive seizures.

An Understanding of Suctioning
By Guardian Angels Training
Enhance respiratory care with our "Understanding Suctioning Techniques and Practices" course. Gain comprehensive knowledge and practical skills for safe and effective suctioning. Evidence-based practices, infection prevention, and patient-centred care emphasised.

An Understanding of Physiological Observations
By Guardian Angels Training
Gain essential knowledge and practical skills to accurately monitor and interpret physiological parameters in patients with our "An Understanding of Physiological Observations" course. Learn to conduct and interpret various observations to support informed clinical decision-making.

An Understanding of Respiratory Care
By Guardian Angels Training
Enhance your respiratory care skills with our comprehensive course. Learn to assess, diagnose, and manage respiratory conditions for better patient outcomes.
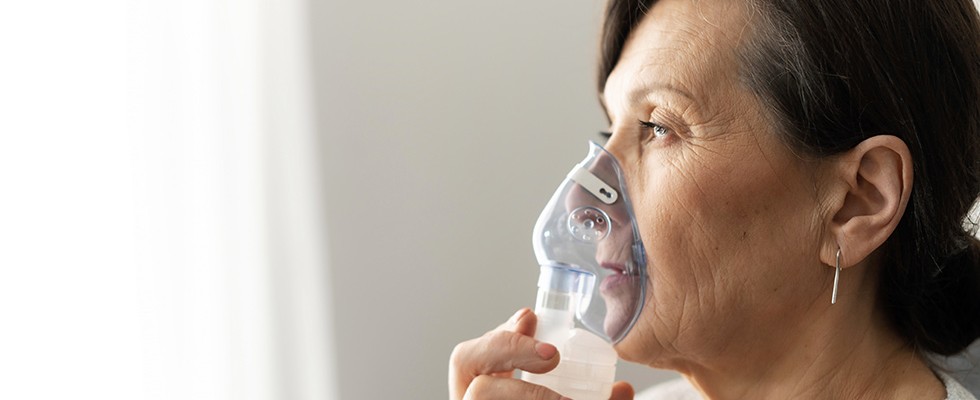
An Understanding of Fine-Bore Nasogastric Tube Insertion, Care and Safe Use
By Guardian Angels Training
Gain comprehensive knowledge and practical skills for safe and effective fine-bore nasogastric tube insertion, care, and use with our healthcare professional course.

An Understanding of Epilepsy and the Simulated Administration of Buccal Midazolam (non-RQF)
By Guardian Angels Training
Gain comprehensive knowledge on epilepsy management and simulated administration of buccal midazolam with our course. Respond effectively to seizure emergencies.

Search By Location
- Nursing Courses in London
- Nursing Courses in Birmingham
- Nursing Courses in Glasgow
- Nursing Courses in Liverpool
- Nursing Courses in Bristol
- Nursing Courses in Manchester
- Nursing Courses in Sheffield
- Nursing Courses in Leeds
- Nursing Courses in Edinburgh
- Nursing Courses in Leicester
- Nursing Courses in Coventry
- Nursing Courses in Bradford
- Nursing Courses in Cardiff
- Nursing Courses in Belfast
- Nursing Courses in Nottingham