- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
7937 Medicine & Nursing courses
A System Approach to Good Pharmacovigilance Practice
By Research Quality Association
Course Information In today's evolving landscape, pharmacovigilance (PV) systems face ongoing challenges due to global, national, and company-specific events. This course focuses on developing personnel equipped to navigate these complexities and improve the safe use of medicinal products. We emphasise continuous global thinking, communication, and strategic planning, ensuring adaptability across various levels. The course explores maintaining PV system functionality while adhering to regulatory requirements. Participants will apply European regulatory standards to enhance and sustain PV system effectiveness, contributing to improvement initiatives and ensuring operational integrity. The course covers PV system intricacies, regulatory compliance, and a 'systems approach' for auditors, quality assurance personnel, and PV practitioners. Through presentations and workshops, attendees gain insights into implementing and maintaining an effective PV system. Who should attend? Auditors Pharmacovigilance Quality System Managers Pharmacovigilance scientists The QPPV. Course benefits Throughout the course delegates will explore application of the legal requirements for the PV system and quality system and how to assure these systems. They will demonstrate their ability to contribute to: A systematic investigation of the pharmacovigilance system and its quality system Examination of how the pharmacovigilance system and quality system interact to achieve compliance. The risk-based approach to auditing the PV system and quality system The maintenance of 'inspection readiness' Explore how to investigate the complex PV system Discussions about how to monitor and maintain the PV system and assure compliance. Course Objectives Clarify what has to be done: Explore application of the legal requirements. Explore how to do what has to be done: Adopt a systemic approach to systematically investigate or implement and maintain the PV system and quality system Examine how a compliant PV system and a compliant quality system interact to achieve compliance with regulatory requirements for PV Explore how to investigate the complexity of the PV system. Discus how to identify what is missing or what needs to be improved: Discuss how to monitor and maintain the PV system and assure compliance. This course will assist delegates with: An understanding of key system principles, A practical approach to implementing, maintaining and monitoring the PV system and its quality system A procedure to share expertise to increase efficiency and confidence. This course is structured to encourage delegates to: Discuss and develop ideas - Share knowledge and experiences - Solve specific problems. By the end of the course delegates will be able to: Understand better the pharmacovigilance system, its quality system and how the components interact to achieve the objectives of pharmacovigilance Investigate, and analyse the pharmacovigilance system and to identify what is missing and what needs to be improved. Tutors Tutors will be comprised of (click the photos for biographies): Jana Hyankova Head of PV Department, IVIGEE Services a.s. Programme Please note timings may be subject to alteration. Day 1 08:30 Welcome, registration, course objectives and introduction to work groups Housekeeping notices, meet other delegates, explore how to work in your work group, course objectives. Clarify the definition and objectives of Pharmacovigilance. 09:30 The Regulatory Framework for Pharmacovigilance Identify the relevant regulations and directives. Explore GVP guidance, structure of the modules and standard format of each module. 10:00 The Pharmacovigilance System Exploration of how to organise what has to be done, communications. Exploration of the structures and processes for pharmacovigilance. 10:30 Break 11:00 Workshop 1 and Feedback Exploring an organisational model of the pharmaceutical company- cooperation between PV and other stakeholders. 12:00 The Quality System for pharmacovigilance Exploration of the structures, processes for the PV quality system and discussion of how it interacts with the pharmacovigilance system to meet the objectives of pharmacovigilance effectively and efficiently. 13:00 Lunch 14:00 Workshop 2 and Feedback The quality system puzzle. Explore the organisation of the PV quality system and how it interacts with the PV system. 14:30 The Quality System for pharmacovigilance Exploration of the structures processes for the PV quality system and discussion of how it interacts with the pharmacovigilance system to meet the objectives of pharmacovigilance effectively and efficiently. 15:00 Description of PV System 15:30 Break 15:30 Workshop 3 and Feedback The quality system puzzle Explore the organisation of the PV quality system and how it interacts with the PV system. 16:00 The Pharmacovigilance Safety Master File Construction of the Pharmacovigilance System Master File and its purpose. 17:00 Workshop 3 and Feedback Description of PV System. 18:00 End of Day Day 2 08:30 Drug Safety in the Clinical Trial Environment - Part 1 Information flow and responsibilities of the sponsor. 09:30 Workshop 4 and Feedback Drug Safety in the Clinical Trial environment: Information flow and responsibilities of the sponsor. 10:30 Break 11:00 Drug Safety in the Clinical Trial Environment - Part 2 Information flow and responsibilities of the sponsor. 12:00 Lunch 13:00 Workshop 5 and Feedback Drug Safety in the clinical trial environment: Information flow and responsibilities of the sponsor. 13:30 Processing of Safety Data Exploration of safety data processing, verification, validation, follow up, formatting and collation, reporting requirements, quality and data management. 15:00 Break 15:30 EudraVigilance Exploration of how EudraVigilance supports the PV system. 16:15 Signal Detection and Evaluation/Risk Benefit Assessment: Pharmacovigilance Risk Assessment Committee (PRAC): What is a signal? What are the regulatory requirements? How is signal detection and evaluation conducted? Qualitative and quantitative methods of signal detection. Risk benefit assessment. 17:00 Risk Management Plans A cornerstone of Pharmacovigilance safety communications, direct healthcare professional communication 18:00 End of Day Day 3 08:30 The Pharmacovigilance Risk Assessment Committee (PRAC) Exploration of how good practice is achieved. Composition, role and responsibilities. Examples of referrals. 09:15 Development Safety Update Reports (DSURs): Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 10:00 Periodic Safety Update Reports (PSURs)/Periodic Benefit Risk Evaluation Reports (PBRERs) Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 10:30 Break 11:00 Periodic Safety Update Reports (PSURs)/Periodic Benefit Risk Evaluation Reports (PBRERs) Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 12:00 Workshop 6 and Feedback To explore the compilation and submission of the PSUR. 13:00 Lunch 13:30 Role of the QPPV Exploration of the legal responsibilities of the QPPV and the MAH. 14:30 Break 15:00 Workshop 7 and Feedback To explore the challenges faced by the QPPV. 15:30 End of course Extra Information Face-to-Face Course Course material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device< Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking You will need a stable internet connection, a microphone and a webcam. CPD Points 23 Points Development Level Develop

Qualsafe Level 4 Certificate in First Response Emergency Care (FREC4)
By Pulse South Coast Ltd - Training & Medical Services
Duration 5 Days Course Overview The Qualsafe Level 4 Certificate in First Response Emergency Care (RQF) stands as the premier qualification for private ambulance providers, encompassing the breadth of skills requisite for serving as an Emergency Care Assistant. Over the course of 5 days, participants will delve into the intricacies of prehospital emergency care, achieving competency aligned with descriptor level E of the PHEM skills framework. An Emergency Care Assistant plays a pivotal role within the UK healthcare landscape, operating as a vital member of ambulance crews, delivering urgent medical aid and facilitating transportation for patients in critical scenarios. This role is also highly esteemed within the events industry. Embark on a transformative journey with the QA Level 4 Certificate in First Response Emergency Care (FREC 4) RQF, a nationally recognised qualification meticulously tailored to cater to aspiring Emergency Care Assistants. This comprehensive curriculum equips participants with the requisite theoretical knowledge and practical skills to navigate a spectrum of prehospital emergency care situations with confidence and proficiency. As the next step of the QA Level 3 Certificate in First Response Emergency Care (RQF), this qualification broadens the horizons of prehospital care practice, unlocking myriad opportunities for career advancement within the NHS, private ambulance services, and specialised roles in the military, police, security, and fire services. Prerequisites Completion of the QA Level 3 Award in First Response Emergency Care (RQF) or equivalent qualification. What's Covered Initial patient assessment Physiological observations Anatomy and physiology Heart disease Electrocardiogram application and monitoring Managing a patient’s airway – supraglottic airways Medical gases – including dosages and administration Thoracic trauma Sepsis Maternity care Respiratory disorders and infections Mechanisms of injury and kinetics Triage Full body and limb immobilisation Sexual assault Major incidents Psychosocial development in children Emergency care for sick and injured children Mental health crisis Assisting the clinician with clinical interventions Course Information Venue: All our 'Open' courses are delivered from our dedicated training venue: Pulse South Coast Ltd, Bentley Wildfowl, Harveys Lane, Ringmer, BN8 5AF On occasion we may use a separate venue, this will be advised on the course booking. Facilities: On your course you will be provided with tea & coffee making facilities and biscuits. We are in a rural location so we recommend brining lunch with you on the day. There is a microwave & fridge on site for your use. There is a garage that is approximately a 10 minute drive from our venue. Parking: We have a dedicated car park that is approximately a 300m walk to the training venue. There is no additional cost to use this parking. We accept no responsibility for any personal loss or theft while you are on our training courses or premises. Please ensure to keep your personal belongings safe at all times. What is required from you: All courses are delivered in English, you must have a basic understanding of the English language to attend. There is a requirement for you to show that you are capable of performing the skills you are taught on the course, due to this you will need to be physically able to demonstrate all practical elements of the course. Dress: As a company we pride ourselves on running immersive & practical training courses. You will often be expected to kneel & work outdoors, please dress appropriately. Identification: As we deliver Ofqual accredited courses, there is a requirement for you to show a form of ID on the first day of the course. This could be; a driving license, passport or something that clearly shows who you are. Timings: All of our courses have a minimum training time of six hours a day, we will often advertise a course as seven hours to include break times. On occasion it is required to go over the scheduled times, however this will always be discussed with the group on the day. Trainer: During your course you will have a designated trainer for the duration. They are there not only to assess for the qualification, but to offer you guidance and support throughout. Security: We accept no responsibility for any personal loss or theft while you are on our training courses or premises. Please ensure to keep your personal belongings safe at all times.

Access to HE Diploma (Medicine)
By Egraduate College
Access to Medicine. Regulated by QAA. Widely accepted by UK universities.

Access to HE Diploma (Healthcare Professions)
By Egraduate College
Access to Nursing and Healthcare Professions. Regulated by QAA. Widely accepted by UK universities.

Apparatus (Equipment) Teacher Training course
By Bodywise Pilates Teacher Training
Pilates Apparatus Equipment Training Pilates Foundation Uk accreditation

Peripheral Intravenous Cannulation (Peripheral vascular access device)
By Guardian Angels Training
Gain the knowledge and skills to safely insert and manage peripheral IV cannulas with our training course for healthcare professionals. Ideal for nurses, medical personnel, and other practitioners.
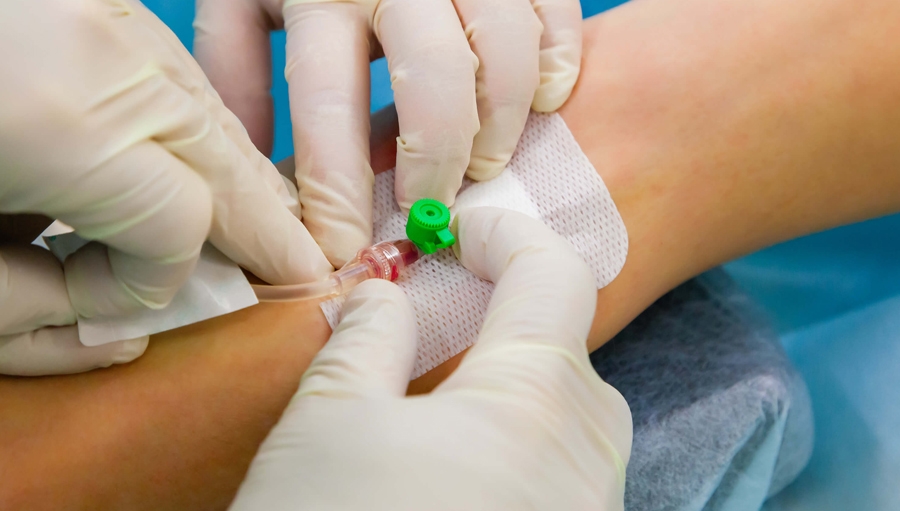
Intravenous Administration of Fluid and Medication
By Guardian Angels Training
Gain the knowledge and skills for safe and effective intravenous therapy with our "Intravenous Administration of Fluid and Medication" course. Ideal for healthcare professionals administering IV fluids and medications.
Access to HE – Level 3 Health & Social Care
By Step Into Learning
Access to HE courses provide a good foundation in the knowledge and skills required for studying at university level, so that students are confident and well prepared when they go on to higher education.

Level 3 Endorsed Award in Delivering Health and Social Care Training (Healthcare Train the Trainer)
By Guardian Angels Training
Gain expertise in healthcare training with our Level 3 Endorsed Award in Delivering Health and Social Care Training. Our comprehensive program equips you with the skills and knowledge to become a proficient trainer in the healthcare sector.

Practical TMJ Therapy Course
By ArchformByte
The Truth About Dr. Truitt Dr Truitt’s decades of expertise merging orthopedics and specialty orthodontics is unrivaled. For over 40 years, he’s been teaching dentists how to become specialists in maxillofacial orthopedics and specialty orthodontics, and he’s graduated over 200,000 students from his programs across 5 continents. He doesn’t just teach his students how to straighten their teeth – he educates them on how to treat patients holistically. He demonstrates how to positively affect overall physical health by focusing on: Complications caused by crowded teeth Airway obstructions Ear way problems He also helps you diagnose and treat patients too, having built out an entire team to support advanced orthodontists on their way to providing unparalleled care. Don’t miss this opportunity to take your practice to the next level

Search By Location
- Medicine & Nursing Courses in London
- Medicine & Nursing Courses in Birmingham
- Medicine & Nursing Courses in Glasgow
- Medicine & Nursing Courses in Liverpool
- Medicine & Nursing Courses in Bristol
- Medicine & Nursing Courses in Manchester
- Medicine & Nursing Courses in Sheffield
- Medicine & Nursing Courses in Leeds
- Medicine & Nursing Courses in Edinburgh
- Medicine & Nursing Courses in Leicester
- Medicine & Nursing Courses in Coventry
- Medicine & Nursing Courses in Bradford
- Medicine & Nursing Courses in Cardiff
- Medicine & Nursing Courses in Belfast
- Medicine & Nursing Courses in Nottingham