- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
7908 Medicine & Nursing courses
Hypnotherapy Practitioner Diploma Course : Feb - March 2025
By Hypnotic Solutions Training
Hypnotherapy Training Course

FAA Level 1 Award In Awareness Of Safeguarding (RQF) Face-to-Face Classroom: Half-day course Virtual Classroom: 2 sessions of 2 ½ hours For those who work with children, young people and vulnerable adults Promotes awareness of safeguarding, enabling learners to identify problems and show where to report these to Course Contents: Safeguarding legislation and guidance Key safeguarding roles Different types of abuse Signs and indicators of abuse and neglect Actions to take when a safeguarding concern arises Benefits of this course: In 2018/2019, 415,050 concerns of abuse were raised In 2018/2019, there were nearly 400,000 children in need 52,300 children were subject to a child protection plan 63% of adult safeguarding concerns are for people over 65 1 in every 42 adults aged 85+ have required safeguarding enquires... Child abuse often goes unreported and unrecorded - till it is picked up on by someone who then does something about it. This Level 1 Safeguarding Awareness course gives people the knowledge to make a real difference to a person's life! This basic Safeguarding course is a nationally recognised, Ofqual regulated qualifications accredited by First Aid Awards Ltd. This means that you can be rest assured that your Level 1 in Safeguarding Certificate provides information for best practice to make a real difference to protect the health and wellbeing of the most vulnerable in our society. The Ofqual Register number for this course is 603/5635/2

Good Laboratory Practice Refresher and Hot Topics
By Research Quality Association
Course Information Join us for a comprehensive refresher focusing on crucial Good Laboratory Practice (GLP) requirements, including an emphasis on data integrity, recent developments, and emerging trends gleaned from MHRA inspections. The programme dives into specific domains such as risk assessment, OECD guidance on sponsor influence, and the advisory from OECD on QA. Additionally, delegates can benefit from a dedicated GLP clinic, facilitating discussions on understanding and upholding GLP compliance. Is this course for you? This course is tailored for study directors, principal investigators, test facility management, and QA professionals seeking to refresh their knowledge and responsibilities within the GLP framework. Tutors Tutors will be comprised of (click the photos for biographies): Vanessa Grant -, - Tim Stiles Consultant, Qualogy Ltd Programme Please note timings may be subject to alteration. Day 1 09:00 Registration, Welcome and Introduction 09:20 Development of Good Laboratory Practice A reminder of the history of GLP, its current scope and application, with a synopsis of current UK, European and international standards. 09:50 Roles and Responsibilities of Study Director, Test Facility Management, Principal Investigator, Test Site Management, Study Staff and QA A reminder of the roles and responsibilities with regard to the GLP management and oversight of the Test Facility and the management and control of the study, as defined by GLP. 10:30 Break 10:45 Workshop 1 Workshop 1 Roles and responsibilities 11:15 Influence of Sponsors The published OECD Position Paper No. 21 regarding Possible Influence of Sponsors on conclusions of GLP Studies is reviewed and discussed. 11:45 Data Integrity The fundamentals of data integrity according to the OECD Guidance No. 22 on Data Integrity is discussed along with the responsibilities of Study Director, Test Facility Management, and study staff in ensuring the integrity of the GLP study data. 12:30 Lunch 13:15 Quality Assurance and GLP OECD Advisory No. 23 (Revision of OECD No.4)- A walk through of the changes to the OECD Guidance on the role and activities of Quality Assurance 13:45 Quality Improvement Tools and GLP The tools that might be considered for GLP and their role and operation when used in Test Facilities- OECD Position Paper No.24 published July 2022 14:15 Workshop 2 Workshop 2 Change control 14:30 Risk Assessment How should we assess risk and how can we use the process to assist in evaluation audit findings? 15:00 Break 15:15 Current hot topics in GLP Explore the current issues in Industry and trends /types of Regulatory inspection findings 15:50 GLP Clinic An opportunity to discuss any other issues regarding understanding and maintaining GLP Compliance. 16:30 Close of Course Extra Information Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 7 Points Development Level Learn

GA Level 4 Award in Epilepsy and Buccal Midazolam Instruction
By Guardian Angels Training
Gain advanced knowledge and practical skills in instructing buccal midazolam administration for epilepsy with our Level 4 Award course.

Pharmaceutical Validation Training Course (ONLINE) – For More Senior Validation, CQV and C&Q Roles
By Getreskilled (UK)
Take our part-time online “Conversion Course into Pharmaceutical Manufacturing“ NO Previous Industry Experience or Science Qualifications Required

Extended Clinical Skills for Support Workers
By Guardian Angels Training
Enhance your clinical expertise with our "Extended Clinical Skills Training" course. Designed for healthcare professionals, this course provides practical skills and knowledge for extended clinical procedures and interventions.

The course provides professional training in acupuncture, diagnosis and traditional Chinese medicine that prepares students for modern professional life. It combines theory and practice to ensure that students are proficient in traditional Chinese medicine and Acupuncture, as well as adequately versed in modern Western medical sciences,. Emphasis is placed on developing students as competent and confident therapists with a caring and compassionate attitude. The course is run on a part-time basis over a period of two years. Classes are held over 10 3-day weekends per year.

Level 4 Dental Practice Management
By Cavity Dental Training
Unlock Your Potential as a Dental Practice Manager with Our Level 4 Course The Level 4 Diploma in Dental Practice Management qualification delivered by Cavity Training has been designed as a standalone qualification or as an apprenticeship qualification for anyone wishing to become a qualified practice manager. Candidates are required to be in a work placement in order to be able to commence the qualification. Learn about the Cavity Training Dental Practice Management course This qualification allows candidates to learn, develop and practice the skills required for employment and a career in Dental Practice Management. The content covers all essential areas of Management and is mapped to the GDC Learning Outcomes for Dental Nurses and relevant National Occupational Standards. This qualification is approved by the GDC. This qualification allows candidates to go on to higher level 5 management courses and expand their management career. Fees This course can be completed as an government funded apprenticeship, through Cavity Training, or as a privately funded course for £3500. You can either pay as a lump sum or alternatively, you can split into 12 instalments. Entry requirements The minimum requirements are level 2 in English and Maths. Structure To achieve the Level 4 Diploma in Dental Practice Management, candidates will need to also successfully complete a final online Knowledge Test. This is a 24 month course, with a final end point assessment. Knowledge Our course is delivered via live training webinars with specialist tutors. Skills and Behaviours You will be appointed a designated Assessor, who will coach you through your qualification and complete regular assessments with you to support you to complete your qualification. You will have weekly contact from your Assessor. How we compare with our competitors? Don't just take our word for it, here is what our staff think Bridget I did my course years ago. It was classroom based one night per week. I think I would prefer to be more ‘hands on’ like it is now. Cavity really are a great company to work for. I truly believe that there expertise will ensure the next generation are amazing! Gina I did mine over an apprenticeship but the company my employer used wasn’t great and I didn’t get much support. Although I passed I can only imagine the length that Cavity have gone to to ensure that the students feel supported. As an employee, its super! Enquire Today

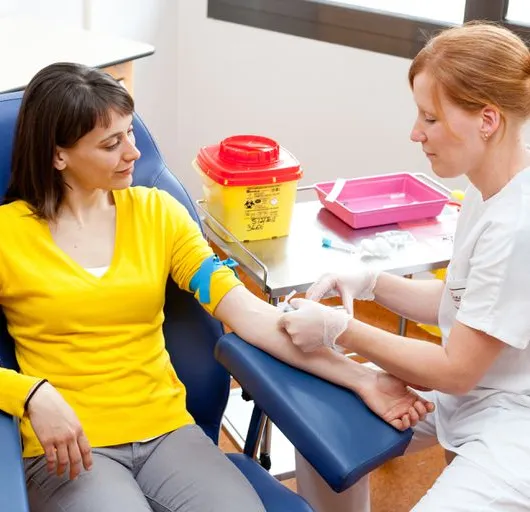
Introduction to Phlebotomy Course (GPT003) - 1 DAY Classroom - Part 1 online + Part 2 Classroom
4.6(39)By Geopace Training
Learn how to take blood ... train as a Phlebotomist Nationally Recognised Qualification No previous experience or qualifications needed OCN Accredited - Level 3 (advanced) CPD Accredited (The CPD Certification Service) Covers all steps up to live blood draw Practise on artificial arm and fake blood! Basic understanding of English language required OPEN TO ALL APPLICANTS
Search By Location
- Medicine & Nursing Courses in London
- Medicine & Nursing Courses in Birmingham
- Medicine & Nursing Courses in Glasgow
- Medicine & Nursing Courses in Liverpool
- Medicine & Nursing Courses in Bristol
- Medicine & Nursing Courses in Manchester
- Medicine & Nursing Courses in Sheffield
- Medicine & Nursing Courses in Leeds
- Medicine & Nursing Courses in Edinburgh
- Medicine & Nursing Courses in Leicester
- Medicine & Nursing Courses in Coventry
- Medicine & Nursing Courses in Bradford
- Medicine & Nursing Courses in Cardiff
- Medicine & Nursing Courses in Belfast
- Medicine & Nursing Courses in Nottingham
