- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
708 Medicine & Nursing courses
Working with Children and the Foundations of PBS
By Guardian Angels Training
Enhance your skills in promoting positive behavior and creating supportive environments for children with our PBS course. Evidence-based practices and collaboration emphasised.

Promoting Best Practice in Basic Life Support Instruction
By Guardian Angels Training
Learn to teach basic life support effectively with our "Promoting Best Practice in Basic Life Support Instruction" course. Ideal for healthcare professionals, educators, and individuals interested in life-saving interventions.

People Handling Instructor and Assessor
By Guardian Angels Training
Gain certification as a safe people handling instructor and assessor with our comprehensive course. Equip yourself with the necessary skills and knowledge to effectively train and assess others in safe manual handling techniques.

Promoting Best Practice in Venepuncture Instruction
By Guardian Angels Training
Enhance your venepuncture skills with our comprehensive course. Learn evidence-based techniques, infection prevention measures, patient-centered communication, and ethical considerations to ensure safe and effective practices.
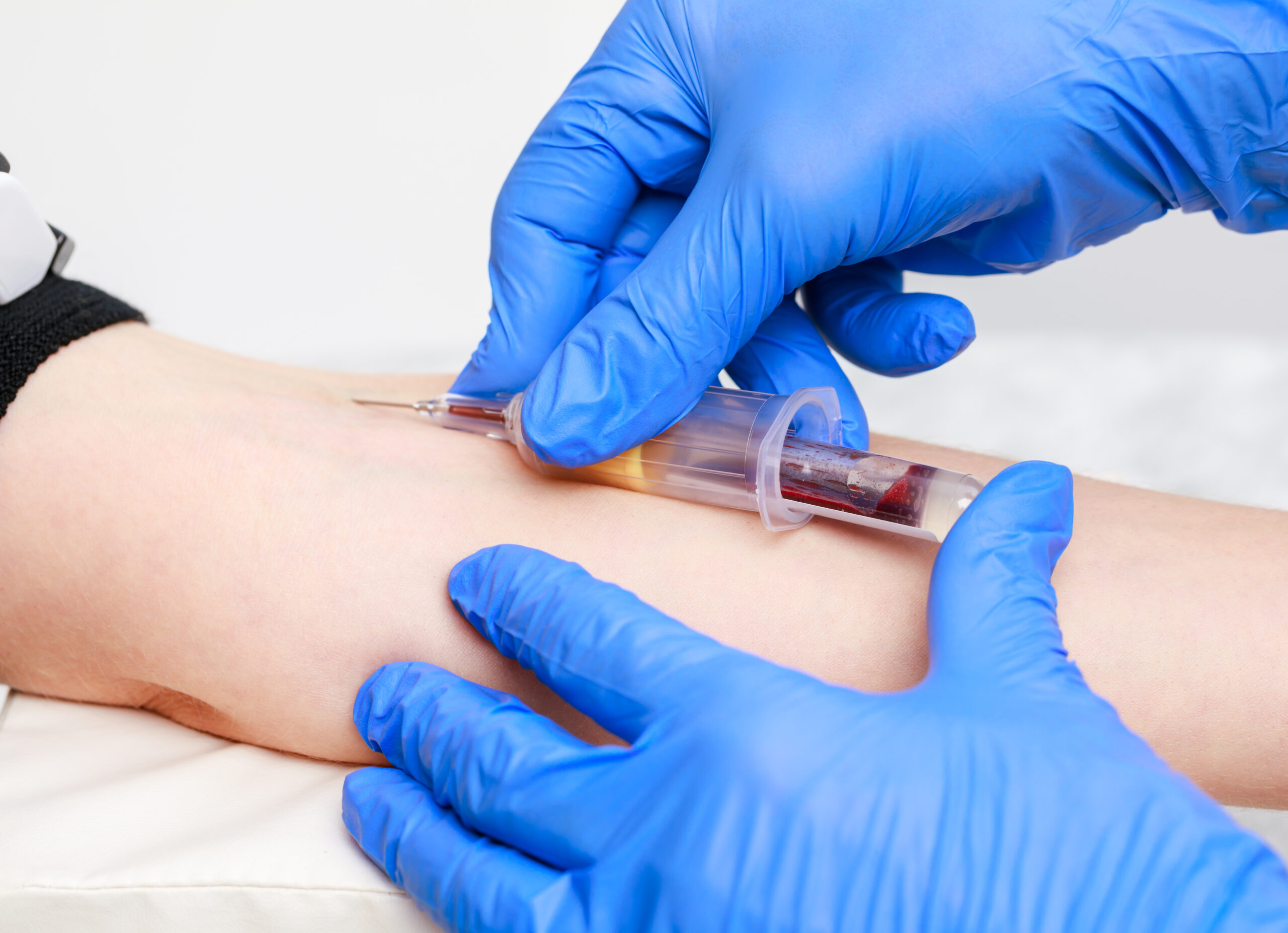
Promoting Best Practice in Asthma and Anaphylaxis Training (Train the Trainer)
By Guardian Angels Training
Gain comprehensive knowledge and practical skills in asthma and anaphylaxis management with our evidence-based training course. Ideal for healthcare professionals, educators, and patient care providers. Optimise patient outcomes with effective emergency response protocols, prevention strategies, and patient education.
Promoting Best Practice in Teaching and Assessing Medicines Management (Medication Train the Trainer)
By Guardian Angels Training
"Empower educators and healthcare professionals with evidence-based teaching strategies and practical assessment methods through our 'Promoting Best Practice in Teaching and Assessing Medicines Management' course. Ensure safe and competent medication administration practices among healthcare learners. Enroll now."
Train the Trainer / Instructor Course in Nasogastric Tube Insertion and Feeding
By Guardian Angels Training
Gain comprehensive knowledge and practical skills for safe and effective nasogastric tube insertion and feeding techniques with our "Promoting Best Practice in Nasogastric Tube Insertion and Feeding Tuition" course. Optimise patient safety, comfort, and outcomes with evidence-based best practices.

An Understanding of Microsuctioning
By Guardian Angels Training
Learn the safe and precise technique of microsuctioning for earwax and foreign object removal with our comprehensive course for healthcare professionals.

University Foundation Programme
By Bath Academy
The University Foundation Programme (UFP) is a one-year intensive course that prepares both British and international students to attend top UK universities.

Clinical Coach Standardisation Event September 2025
By Samantha Morgan-Hourd
Clinical coach standardisation events

Search By Location
- Medicine & Nursing Courses in London
- Medicine & Nursing Courses in Birmingham
- Medicine & Nursing Courses in Glasgow
- Medicine & Nursing Courses in Liverpool
- Medicine & Nursing Courses in Bristol
- Medicine & Nursing Courses in Manchester
- Medicine & Nursing Courses in Sheffield
- Medicine & Nursing Courses in Leeds
- Medicine & Nursing Courses in Edinburgh
- Medicine & Nursing Courses in Leicester
- Medicine & Nursing Courses in Coventry
- Medicine & Nursing Courses in Bradford
- Medicine & Nursing Courses in Cardiff
- Medicine & Nursing Courses in Belfast
- Medicine & Nursing Courses in Nottingham