- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
9635 ET courses
Electrical Power Failure Analysis and Investigations – Insightful Investigations For Precision Resolutions
By EnergyEdge - Training for a Sustainable Energy Future
Enhance your expertise in electrical power failure analysis and investigations with EnergyEdge's insightful course for precision resolutions. Enroll now!

Casino Croupier course
By Ace Academy
Learn everything that you need to know to become a casino croupier then decide whether you want to work on cruise ships or casinos all over the world

Start your career as a Cyber Security Manager/ Engineer and earn upto £35,000/ Month Are you ready to be the first line of defence in the digital world? Ever dreamed of becoming the unsung hero who safeguards the virtual corridors of data and privacy? Welcome to our Cyber Security Job Placement course, a cutting-edge, comprehensive program designed not only to equip you with the crucial skills required in the fast-paced realm of cybersecurity but also to give you that much-needed edge in today's competitive job market. Our course promises more than just theoretical knowledge. By merging an industry-driven curriculum with real-world scenarios, we prepare you to tackle the most formidable security challenges. Our robust Career Support guarantees individualised mentorship and networking opportunities with the industry's top professionals. But the cherry on top? Our Job Ready Program - we don't just teach you how to fish; we ensure you catch your big one! So, are you ready to take the plunge into the exciting world of cybersecurity? Secure your future with us today because in our world, opportunity doesn't knock twice! Our mission is simple - to be your trusted partner every step of the way, from training to employment. In addition to teaching you the technical skills you need, we will also provide you with career mentoring and support. We will help you build your resume, prepare for interviews, and land your dream job. We also have partnerships with many companies that are hiring IT Network Cyber Security, so we can help you get your foot in the door. If you are not happy with our service, we also offer a 100% money-back guarantee. So what are you waiting for? Enrol in our IT Network Cyber Security Training Program today and start your journey to becoming a successful IT Network Cyber Security Manager/ Engineer! If you have any questions, you can contact us. We will be happy to provide you with all the information you need. Why Choose Our Cyber Security Engineer Job Placement Program? So, what sets us apart from other programs? Let's dive into the exceptional benefits you'll experience when you join our IT Network Cyber Security Training: One-On-One Consultation Sessions with Industry Experts: Gain invaluable insights and guidance from seasoned professionals who have thrived in the IT Network Cyber Security field. Our consultation sessions provide you with insider tips, tricks, and advice, empowering you to navigate the industry with confidence and expertise. Extensive Job Opportunities: We have established partnerships with numerous companies actively seeking Cyber Specialists. Through our network, we'll connect you with exclusive job openings that are not easily accessible elsewhere. Our aim is to maximise your employment prospects and provide you with a range of exciting opportunities to choose from. Interview Preparation: We provide you with access to a comprehensive database of potential interview questions curated over years of industry experience. Walk into your interviews confident, well-prepared, and ready to impress. Money-Back Guarantee: Your satisfaction is our top priority. We are confident in the quality of our training and support, which is why we offer a 14-day money-back guarantee. Continuous Career Support: Our commitment doesn't end when you secure a job. We'll be there for you throughout your career journey, offering continued support and guidance. Whether you need advice on career advancement, assistance with new projects, or simply a friendly ear to share your achievements, we'll be your trusted partner for long-term success. Here are the courses we will provide once you enrol in the Cyber Security Engineer program: Course 01: Cyber Security Incident Handling and Incident Response Course 02: Cyber Security Advanced Training Course 03: Linux File System Course 04: IT Support Technician Course 05: Functional Skills IT Course 06: CompTIA Security+ (SY0-601) Course 07: CompTIA A+ (220-1001) Course 08: CompTIA A+ (220-1002) Course 09: Cloud Computing / CompTIA Cloud+ (CV0-002) Course 10: Learning Computers and Internet Course 11: CompTIA CySA+ Cybersecurity Analyst (CS0-002) Course 12: CompTIA IT Fundamentals ITF+ (FCO-U61) Course 13: CompTIA Network (N10-007) Course 14: CompTIA PenTest+ (Ethical Hacking) Course 15: Cryptography These courses will help you to develop your knowledge and skills to become a successful Cyber Security Expert. Cyber Security Engineer Job Placement Program The Cyber Security Engineer Job Placement Program is completed in 9 easy steps: Step 1: Enrol in the Cyber Security Engineer Job Placement Programme Begin your exciting journey with us by enrolling in the IT Network Cyber Security program. Complete your registration and make a secure online payment. Remember, we offer a 14-day money-back guarantee if you're not completely satisfied. After you enrol in the Program, you will get lifetime access to 15 premium courses related to IT Network Cyber Security. These courses will teach you the knowledge and skills required to become a successful Cyber Security expert. Our customer service team will help you and keep in contact with you every step of the way. So you won't have to worry about a thing! Step 2: Initial One-On-One Counselling Session Once enrolled, you will be paired with a dedicated career mentor. Schedule your first one-on-one session to discuss your career aspirations, skills, experience, and any areas for potential growth. This conversation will shape your learning and development path. Step 3: Certification upon Course Completion After learning from the courses, you must obtain certificates for each course. There will be exams for every course, and you have to pass them to get your certificate. To pass successfully, you must get 90% marks. Once you pass the exams, you will receive hardcopy certificates. These certificates will prove that you're an expert in the subject. Step 4: CV Revamping Our team of professionals will build you a compelling CV and LinkedIn profile. We'll ensure it presents your skills and qualifications effectively and is tailored to the needs and expectations of the Cyber Security - IT industry. With these powerful tools in hand, you'll be fully prepared to tackle job interviews confidently. Step 5: Building Network and Submitting CV We understand the power of casting a wide net. We'll strategically submit your CV to various platforms and networks, expanding your reach and connecting you with valuable opportunities that align with your career goals. We will also make connections with many high-profile individuals and companies through your LinkedIn profile. Step 6: Interview Preparation With your CV ready, we'll move on to interview preparation. Gain exclusive access to our database of potential interview questions. Through simulated interviews with your mentor, you'll practice your responses and receive valuable feedback to further refine your skills. Step 7: Securing Job Interviews Leveraging our partnerships with leading companies, we'll secure job interviews for you. We'll ensure you get the opportunity to showcase your skills to potential employers and get the dream job you want. Step 8: Post-Interview Support Post-interview, we'll provide a debriefing session to reflect on your performance and identify areas of improvement for future interviews if necessary. Remember, our commitment extends until you land your dream job. Step 9: Celebrate Your New Job! Once you've secured your dream job in IT Network Cyber Security, it's time to celebrate! However, our support doesn't end there. We'll provide you with ongoing career advice to ensure you continue to thrive in your new role. We're excited to accompany you on this journey to success. Enrol today, and let's get started! Your path to a successful career in IT Network Cyber Security begins with us. So enrol now in thisCyber Security Engineer Job Placement Program to kickstart or uplift your career! CPD 100 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Cyber Security Engineer Job Placement Program Cyber Security Engineer Job Placement Program This Cyber Security Engineer Job Placement Program course is for individuals who are: IT professionals looking to specialise in cybersecurity. Graduates in computer science or related fields seeking entry into the cybersecurity domain. Career changers who are intrigued by the prospect of a career in the ever-evolving IT security field. Anyone interested in understanding how to protect systems and data from cyber threats. Requirements Cyber Security Engineer Job Placement Program No experience is required to enrol in Cyber Security Engineer Job Placement Program. Just enrol in the Cyber Security Engineer Job Placement Program & start learning. Career path Cyber Security Engineer Job Placement Program Cybersecurity Analyst: £30,000 - £50,000 Information Security Specialist: £45,000 - £65,000 Cybersecurity Engineer: £55,000 - £80,000 Cybersecurity Manager: £65,000 - £95,000 Cyber Security Manager £45,000 - £90,000 Cyber Security Trainee £24,000 - £35,000 Cyber Security Analyst £60,000 - £95,000 Certificates CPD Accredited e-Certificate Digital certificate - Included CPD Accredited Framed (Hardcopy) Certificate Hard copy certificate - Included Enrolment Letter Digital certificate - Included QLS Endorsed Hard Copy Certificate Hard copy certificate - Included Student ID Card Digital certificate - Included

PYTHON BOOTCAMP: This 12-week Python Data Analytics Data Boot Camp is designed to give you a complete skill set required by data analysts . You will be fully fluent and confident as a Python data analyst, with full understanding of Python Programming. From Data, databases, datasets, importing, cleaning, transforming, analysing to visualisation and creating awesome dashboards The course is a practical, instructor-lead program.

An Understanding of Male Catheterisation
By Guardian Angels Training
Gain comprehensive knowledge and practical skills for safe and effective male catheterisation with our course. Learn techniques, considerations, and patient care for addressing urinary retention, bladder dysfunction, and monitoring urinary output.
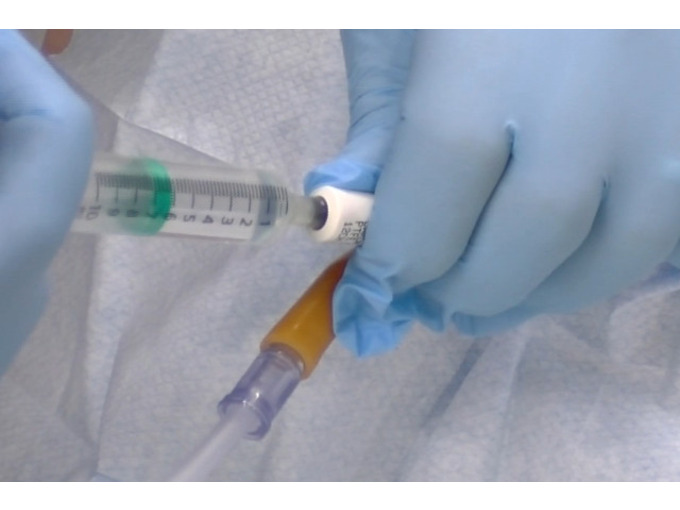
BOHS P402 - Surveying and sampling strategies for Asbestos in buildings
By Airborne Environmental Consultants Ltd
Asbestos surveyors, or managers of surveyors and surveying teams. Asbestos re-inspectors or anyone that undertakes asbestos re-inspections. Those who require a detailed understanding of asbestos surveying principles (e.g. asbestos report writers, architects, building surveyors etc.) Prior Knowledge and Understanding Candidates for this course are expected to be aware of the contents of HSG 264 Asbestos, the survey guide and have a minimum of six months prior experience of assisting on asbestos surveys. In addition, candidates are expected to have had training to cover the core competencies outlined within the foundation material detailed within Table A9.1 of HSG248 Asbestos: The Analysts' Guide (July 2021). This may be achieved by In -house learning or through the P400 foundation module.

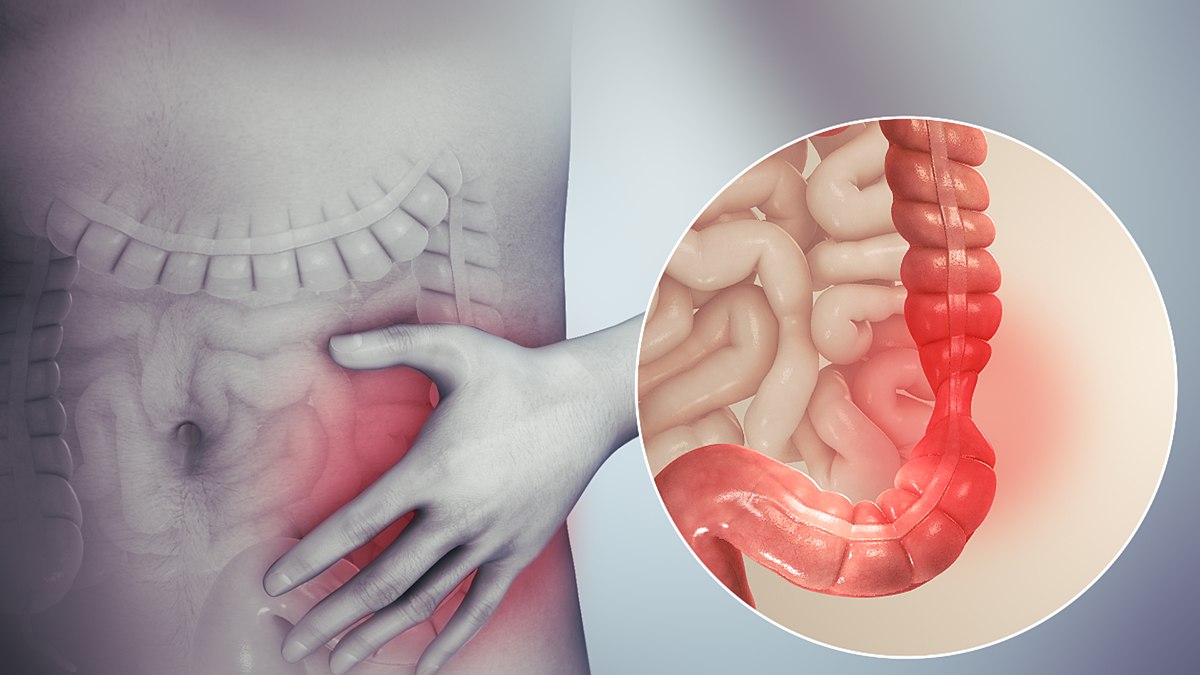
An Understanding of Bowel Care
By Guardian Angels Training
Gain comprehensive knowledge and practical skills for safe and effective bowel care management with our "An Understanding of Bowel Care Techniques" course. Ideal for healthcare professionals seeking to promote patient comfort and prevent complications.
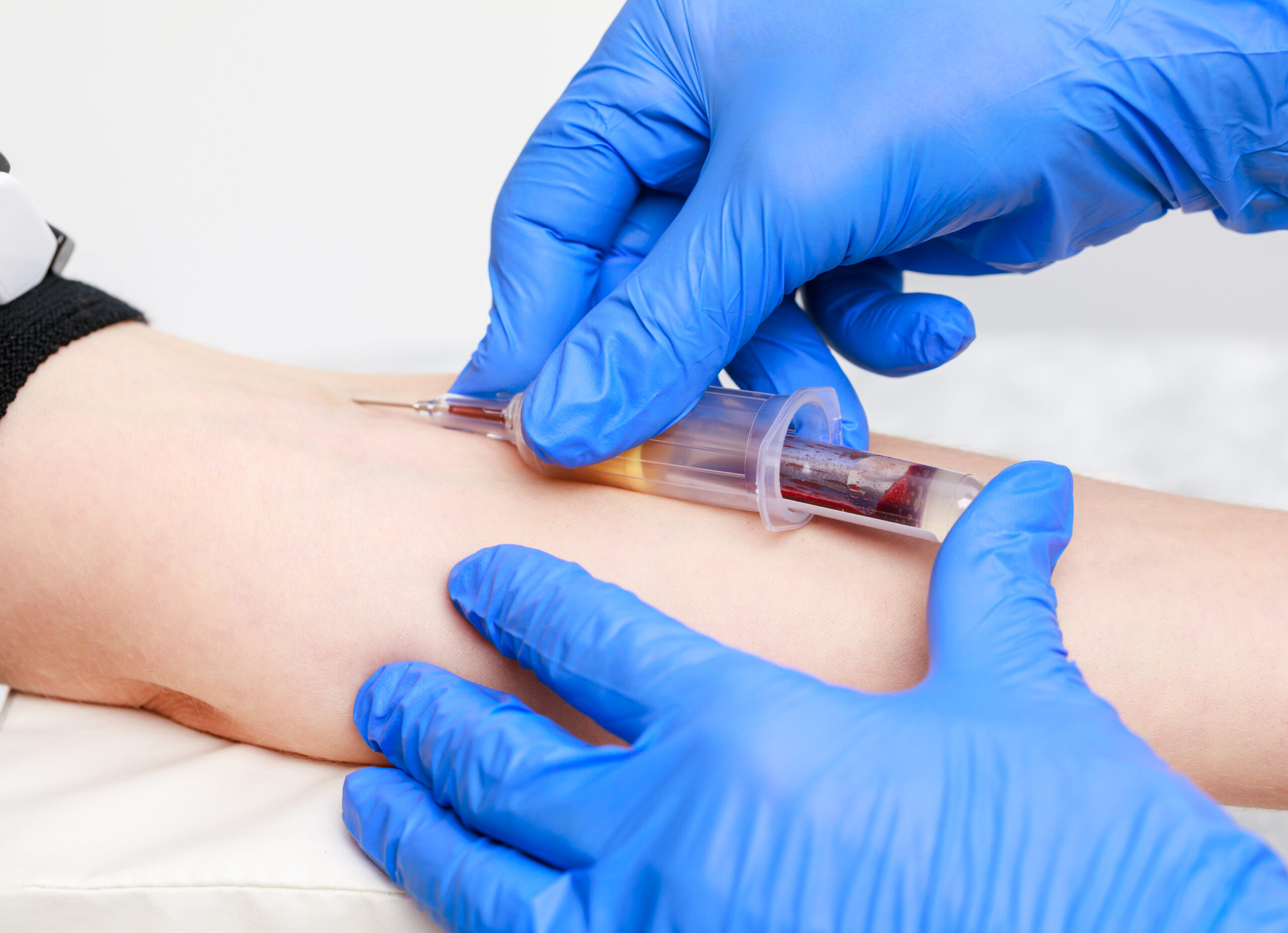
An Understanding of Venepuncture (Adult or Child as applicable)
By Guardian Angels Training
Gain comprehensive knowledge and practical skills for safe and effective venepuncture procedures in adult patients with our "Understanding Adult Venepuncture Techniques" course. Perfect for healthcare professionals seeking to confidently perform venepuncture with accuracy and patient comfort.
Recognising and Responding to Acutely Unwell Individuals
By Guardian Angels Training
Gain the knowledge and skills to identify acute illness in patients with our "Recognising and Responding to Acutely Unwell Individuals" course. Improve patient outcomes and prevent deterioration in various healthcare settings. Enroll now.

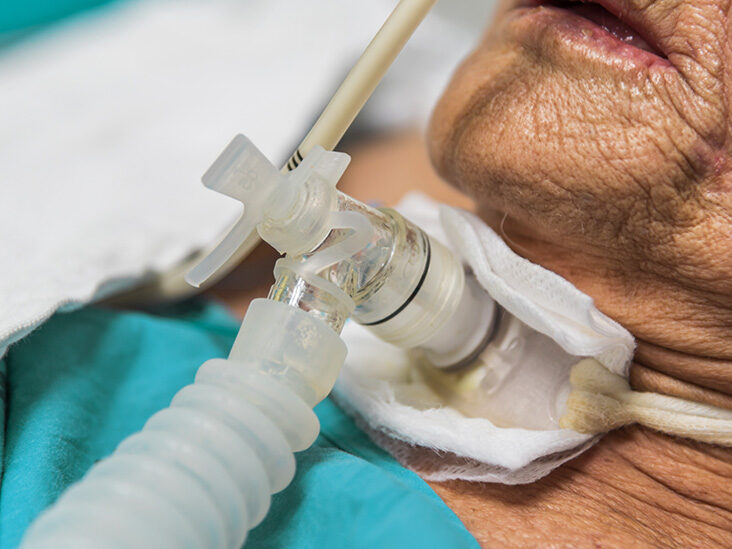
An Understanding of Tracheostomy Care
By Guardian Angels Training
Gain comprehensive knowledge and practical skills to provide safe and effective care for tracheostomy patients with our "Understanding Tracheostomy Care" course. Learn the management and maintenance of surgical openings in the trachea to facilitate breathing.
Search By Location
- ET Courses in London
- ET Courses in Birmingham
- ET Courses in Glasgow
- ET Courses in Liverpool
- ET Courses in Bristol
- ET Courses in Manchester
- ET Courses in Sheffield
- ET Courses in Leeds
- ET Courses in Edinburgh
- ET Courses in Leicester
- ET Courses in Coventry
- ET Courses in Bradford
- ET Courses in Cardiff
- ET Courses in Belfast
- ET Courses in Nottingham