- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
29005 Development courses delivered Online
SOLD OUT - September 2025 Fundamentals Organisation & Relationship Systems Coaching Training
By CRR UK
CRRUK equips professionals with the concepts, skills and tools to build conscious, intentional relationships, and to coach relationship systems of any size.

Tue 9 Sep 2025 - Retail Networking
By Hospice Income Generation Network
Sharing & Networking - Hospice Retail Session aimed at those in retail leadership roles: directors, heads of, deputy heads of THIS SESSION WILL NOT BE RECORDED

Front-line communication is crucial in shaping the public perception of every organisation. This course helps delegates organise their ideas and communicate clearly and effectively through email. By enhancing written communication skills, delegates will become more concise, communicate with conviction, and foster meaningful dialogue. These skills are essential for professionals interacting with the public, as improved communication boosts customer satisfaction. Delegates will learn how to: Write clearly and professionally, enhancing public perception of expertise. Communicate more efficiently in writing, reducing wasted time and increasing profitability. Craft concise, rapport-building messages for internal & external customers, fostering productivity and a positive workplace environment. TOPICS COVERED: Benefits and structure of email; Determining email purpose and planning; Writing effective subject lines and paragraphs; Including relevant details and summarising problems; Proper use of CC and BCC fields; Avoiding common email pitfalls; Proofreading techniques. COURSE FORMAT: This Email Etiquette course is a 90-minute interactive virtual session. Upon registration, delegates will receive online login instructions prior to the class date. This format allows delegates to enhance their skills conveniently from their home or office while improving their professional communication abilities.

In times of crisis, people are in reaction mode. They work more from “fight vs. flight” than by using reason. When we serve these customers, we need to respond appropriately and help them to achieve emotional control so that we can work to solve problems. In this interactive online class, you will learn the tools needed to get yourself in emotional control. You will also learn why people react on emotion and tools to help them get back in control, too. Attendees will be able to: Focus on others to project a UB4ME, customer-centered attitude; Empower people with respect (acknowledging) and control (offering choices); and Understand customers better and develop emotionally-intelligent methods for managing their fears and reactions. Register for this class and you will be sent ONLINE login instructions prior to the class date.
Learners will be introduced to EAS as part of the fire safety solution for tall residential properties. This CPD course provides learners with an understanding of the requirements of BS 5839-1 in relation to, design, installation, commissioning, and maintenance of EAS.

Wed 10 Sep - Light up a Life marcomms
By Hospice Income Generation Network
Sharing & Networking - Light up a Life (or name used) marketing & communications Session aimed at those working in marcomms, as well as whoever else works on the organising of your campaigns and events. THIS SESSION WILL NOT BE RECEORDED

ONLINE CLASS: FOCUSES ON HARASSMENT IN THE WORKPLACE AND HOW TO ADDRESS THE PROBLEM. Harassment doesn’t have to be of a sexual nature – it can include offensive remarks about a person’s gender. For example, anyone could be guilty of harassing another if offensive comments are made about certain group in general. Simple teasing or offhand comments might not be illegal, but harassment is illegal when it creates a hostile or offensive work environment. This workshop teaches how to recognize, understand, and respond to harassment so that you can help to maintain a happy work environment, free from hostility and discomfort. Topics: Harassment Laws: The definition of sexual harassment and quid pro quo. Types of Harassment: Verbal, Non-Verbal, Physical, Psychological (teasing and offhand comments). Creating a happy work environment free from hostility and discomfort. Reporting: What to do when employees witness or experience harassment. Responding: How managers can identify and respond to prohibited conduct. Attendees will be able to: Identify the signs of Harassment and take steps to prevent it, Follow a legal and ethical protocol in responding and reporting it when it is perceived to occur, Involve the correct individuals in being made aware of the occurrence, and Respond appropriately to the situation after it occurs. This workshop is offered in two versions – management-only and staff-only. Online Format—Workplace Professionalism is a 4-hour interactive virtual class. Register for this class and you will be sent ONLINE login instructions prior to the class date. It was an extreme pleasure to have you as part of the Communication Enhancement Training Program. Your presentation was excellent and well received by the staff. Courtney C. Crouch, Jr., PresidentSelected Funeral and Life Insurance Company

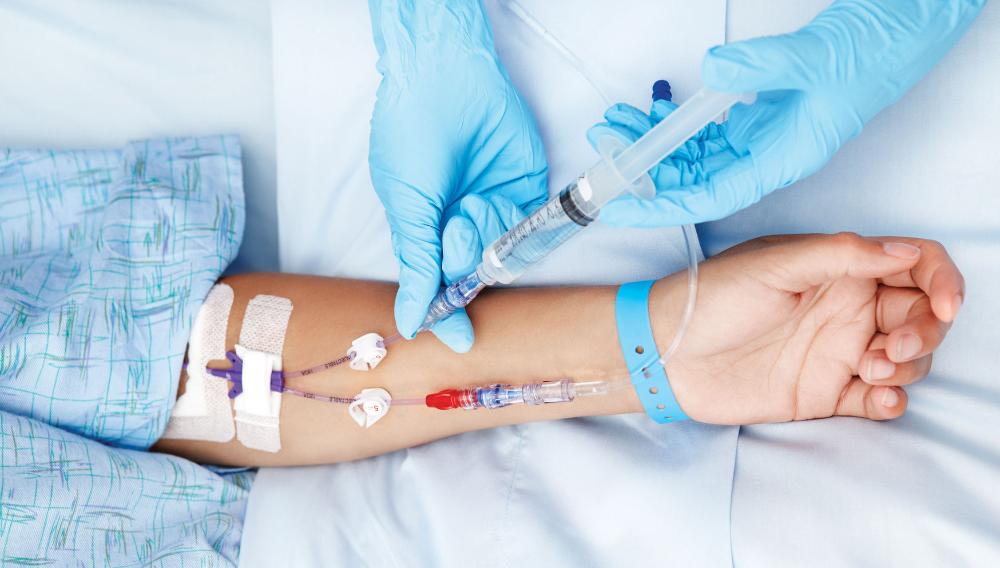
Learn how to cannulate ... Nationally Recognised Qualification OCN Accredited - Level 3 (advanced) - Ireland Level 5 CPD Accredited - The CPD Certification Service Classroom or Virtual Classroom options Covers all steps for cannulating in arm or hand Practise on artificial arms and fake blood! Essential qualification for all IV therapies Phlebotomy training desirable but not essential Basic understanding of English language required OPEN TO ALL APPLICANTS
Quick Summary Fire safety needs to be taken seriously at all times This course is great for an annual refresher of pre-existing training This short course will give you the skills and knowledge to protect your home and work environment from general fire safety hazards Duration: 2 hrs Run via Virtual Classroom Please bear with us while we update this webpage. If you would like more information, please contact us!

Fri 12 Sep 2025 - Newcomers Networking
By Hospice Income Generation Network
Sharing & Networking - Newcomers Networking Session aimed at those who have joined the hospice sector in the last 12mths - all roles and levels. THIS SESSION WILL NOT BE RECORDED
