- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
118 Courses
Welcome to my class! My name is Liz and I teach a gentle and mindful yoga class, open to all. My aim is to help you unwind in the evening and reconnect with your body and inner alignment. The class will be varied and include some slower paced flows (options always available to suit your practice/body), some meditative aspects, and some longer held yin and restorative poses. This is a practice meant for you, so I welcome any feedback/suggestions at the end of my classes with the aim of tailoring my teaching for the greatest benefit of the community. A bit about me: I have been participating in mindful movement for my entire life including experience in classical ballet, partner dance styles, and yoga. My teacher training was completed under a Zen Monk (Daizan Skinner) so I am greatly influenced by these traditions in my teaching. I feel a huge sense of excitement to be sharing this practice so that more people can experience the joy of connecting deeply with themselves, and with life. Outside of yoga, I love nature, music, and crafty activities! I use a tiered pricing system ranging from 5-9 pounds a class Please use whichever option is most appropriate for your unique situation. If you are new to my class, please feel free to take advantage of the newcomers option which is free. Any questions; contact me at: 07539207289 or lizdyeryoga@outlook.com

LOOKING FOR: MG, YA, ADULT FICTION Originally from the North East of Scotland. Kirsten joined Zeno in the summer 2024 after time working at a previous literary agency and before that working as a bookseller in the Charing Cross branch of Foyles. Kirsten has a BA (Hons) in English from Edinburgh Napier University and a Masters in Children’s Literature from Reading University. Kirsten is currently building her own list alongside handling Zeno’s translation rights. Kirsten is looking for: Scottish voices - especially something from the North East - Kirsten is Aberdonian and would love to help a writer from this space emerge. Water horror - as Kirsten grew up in Aberdeen she is always looking for horror inspired by the sea or the landscape of Scotland - bonus points if it has anything to do with an oil rig! Workplace horror like Camilla Grudova’s Children of Paradise or Calvin Kasulke’s Several People are Typing (this is not a reflection of her agency!) Romance - especially young and contemporary - obviously bonus points if it’s set in Scotland and written by someone who has lived there. Kirsten is a huge fan of all the usual suspects, Ali Hazlewood, Ana Huang, Bolu Babalola to name a few! Romantasy - less elves, more out of the box - Kirsten read Sarah J Maas in 2014/15 and so is looking for something that expands on the genre. A good crime thriller - Kirsten did her dissertation on terrorism so anything that is well written in this space would be a dream for her. Additionally she really enjoys serial killer novels and the recent fake true crime novels, Penance by Eliza Clark especially thrilled her. A Studio Ghibli esque fantasy - luscious landscapes, brilliant characters, a powerfulstory. If any of your comps are a Ghibli film she wants to see it! Fanfiction writers! Kirsten has always been on the sites and would love you to tell her if you’ve written fanfiction in the past / have an ongoing fanbase - maybe she has already read it. Kirsten already has: A nonfiction baking book - Kirsten would not want another at this moment. A middle grade cosy fantasy - Kirsten would love to see more of this! But worries if it’s too close to what she already has, then it would be a reason for her to turn it down. A Horror Vampire novel - again she would love to see more of this! But worries if it’s too close to what she already has, that would be a reason for her to turn it down. Vampires would probably be better for her if they were contemporary or in the romantasy space. A Game of Thrones esque fantasy - a client is already writing this and she wants to give it the space on her list it deserves! Kirsten is NOT looking for: Picture books / 5-8 books Poetry Police procedural Hard science fiction Kirsten would like you to submit a covering letter, one page synopsis and the first three chapters (Max 5000 words) of your completed manuscript in a single word document. (In addition to the paid sessions, Kirsten is kindly offering one free session for low income/under-represented writers. Please email agent121@iaminprint.co.uk to apply, outlining your case for this option which is offered at the discretion of I Am In Print). By booking you understand you need to conduct an internet connection test with I Am In Print prior to the event. You also agree to email your material in one document to reach I Am In Print by the stated submission deadline and note that I Am In Print take no responsibility for the advice received during your agent meeting. The submission deadline is: Friday 12th September 2025

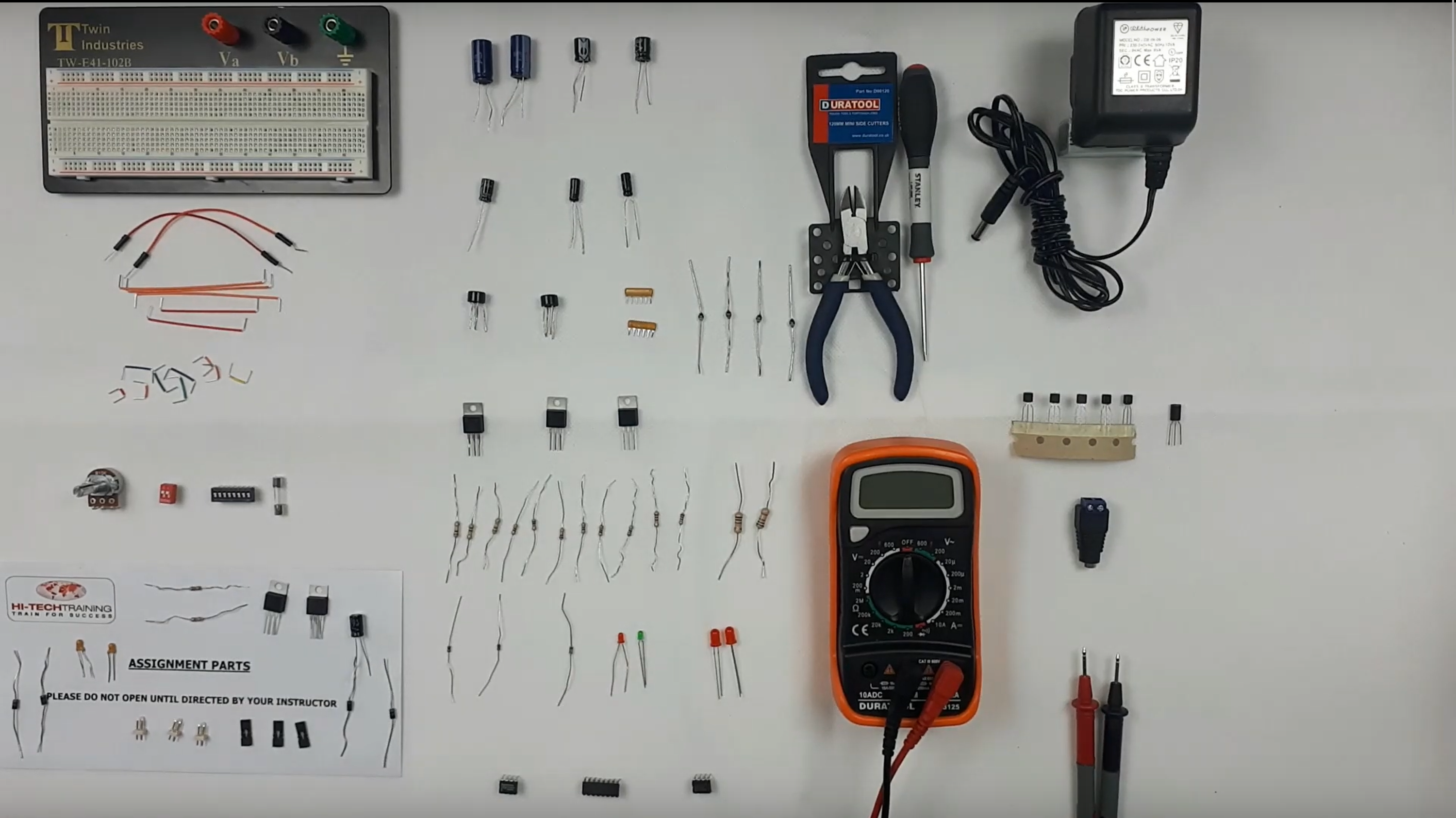
Electronics Repair 2 Course
By Hi-Tech Training
Electronics Equipment Repair 2 (Online Virtual Classroom) is a follow on course to Electronics Equipment Repair 1. This course builds on the skills gained and enables participants to build, test and fault-find more complex Analogue Electronic circuits
Foundations of Yoga: Poses, Posture & Practice Training
By Compete High
When life gets chaotic, you need something stable—something grounding. Foundations of Yoga: Poses, Posture & Practice Training is the answer. Thoughtfully designed for those craving calm, balance, and clarity, this training isn’t about flexibility or fitness—it’s about finding stillness in motion and purpose in your presence. With no deadlines and no demands, Foundations of Yoga: Poses, Posture & Practice Training is your chance to reset on your own terms. And with limited-time access pricing, now is the perfect time to start building your foundation before this offer vanishes. Description You don’t need to be “zen” to begin. You just need a reason to try. Foundations of Yoga: Poses, Posture & Practice Training gives you a fresh, self-paced framework for self-growth, grounded in simplicity and mindfulness. Whether you're chasing calm in chaos or seeking space to breathe, this course is the toolkit you didn’t know you needed. Foundations of Yoga: Poses, Posture & Practice Training puts the pressure-free approach back into personal development. You’ll feel more connected, less anxious, and more resilient—with as little as 5 minutes a day. No strain, no shame, no stress. And yes, you’ll finish what you start—because Foundations of Yoga: Poses, Posture & Practice Training was designed to keep you going, even on your busiest days. Benefits ✅ True self-paced design — follow Foundations of Yoga: Poses, Posture & Practice Training at your own speed. ✅ Gentle and restorative — no pressure, just progress. ✅ Designed for balance — create a space in your day that’s truly yours. ✅ Built for clarity — ease mental clutter with every practice. ✅ Incredible value — skip the studio and save big while gaining lifetime benefits. ✅ No guilt, no grind — just small daily steps toward feeling better. ✅ Make it your ritual — with Foundations of Yoga: Poses, Posture & Practice Training, even a few minutes can transform your day. FAQ Q: Do I need to be fit or flexible to take this course? Not at all. Foundations of Yoga: Poses, Posture & Practice Training is for anyone ready to start from the ground up. Q: Is this really beginner-friendly? Absolutely. Even if you've never rolled out a mat before, Foundations of Yoga: Poses, Posture & Practice Training will make you feel right at home. Q: What if I don’t have time every day? That’s okay! This training is flexible. Foundations of Yoga: Poses, Posture & Practice Training lets you dip in whenever life allows. Q: How long do I have access to the course? Once enrolled, it’s yours. Forever. Come back to Foundations of Yoga: Poses, Posture & Practice Training anytime you need a reset. N:B: This is not a Naked Yoga Course

CompTIA A+ Certification Core 2 (220-1102)
By Packt
This comprehensive course is designed to prepare you to take and pass the CompTIA A+ Certification Core (220-1102) certification exam. This is the second of a two-course series. To become fully A+ certified, you will need to pass both the 220-1101 and 220-1102 exams.

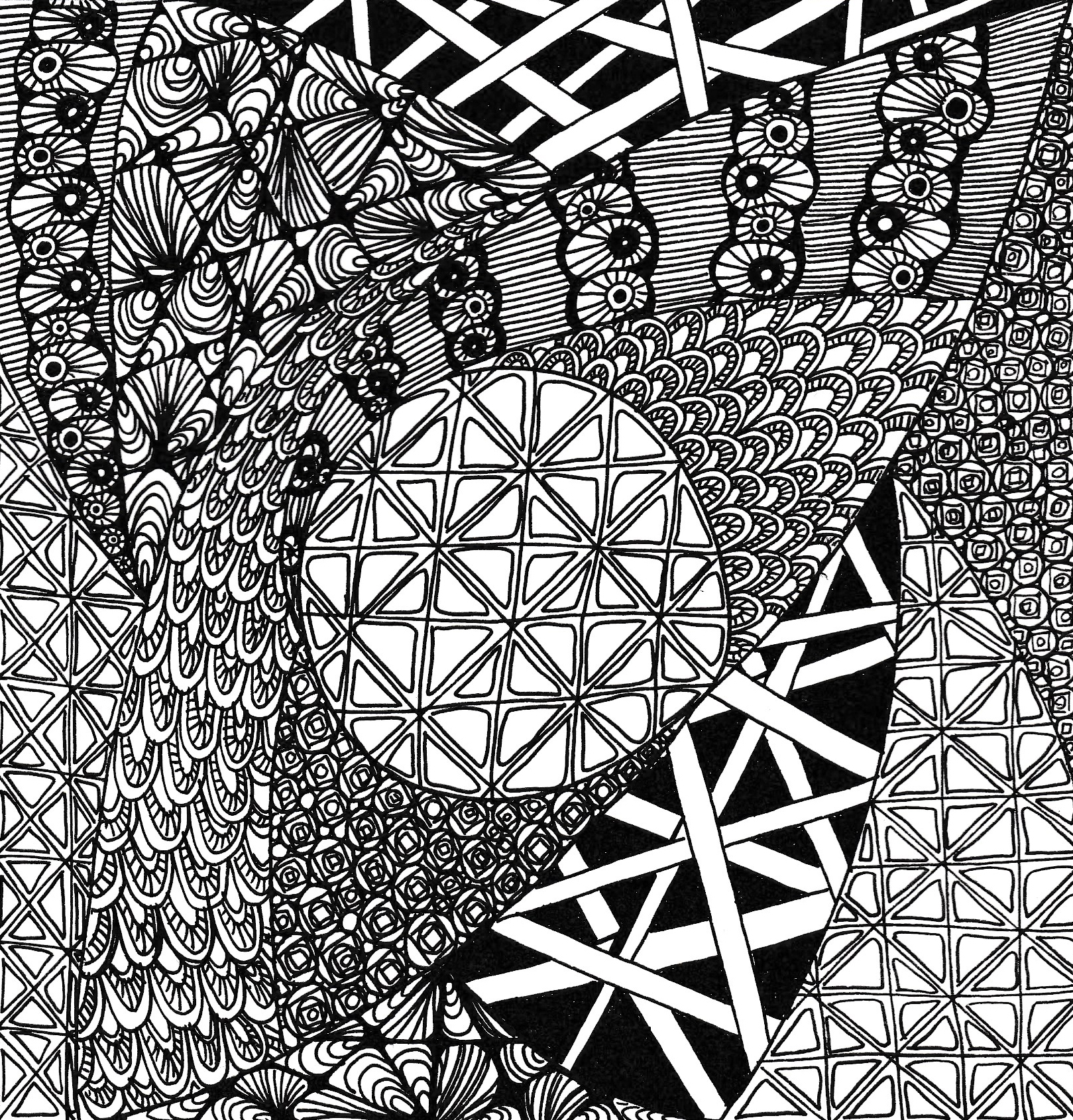
Zenjangle Doodling with Alison
By The Craft Loft
Zenjangle doodling is easy-to-learn, relaxing, and fun and it allows you the freedom to create beautiful images by drawing structured patterns that are a combination of dots, lines, and simple curves.
Computer System Validation Training Course (ONLINE). Extend Your Role to CSV Projects. Get Certified and Become a CSV Professional
By Getreskilled (UK)
Has the Computer System Validation Engineer left and you’ve been handed their responsibilities? Do the thoughts of your next audit fill you with dread? CSV can be frustrating but this program will show you how to manage electronic data in a regulated manufacturing/laboratory/clinical environment using the GAMP framework and ensure compliance with FDA’s 21 CFR Part 11, EU Annex 11 or other regulatory guidelines.

Pharmaceutical Validation Training Course (ONLINE) – For More Senior Validation, CQV and C&Q Roles
By Getreskilled (UK)
Take our part-time online “Conversion Course into Pharmaceutical Manufacturing“ NO Previous Industry Experience or Science Qualifications Required

Search By Location
- Zen Courses in London
- Zen Courses in Birmingham
- Zen Courses in Glasgow
- Zen Courses in Liverpool
- Zen Courses in Bristol
- Zen Courses in Manchester
- Zen Courses in Sheffield
- Zen Courses in Leeds
- Zen Courses in Edinburgh
- Zen Courses in Leicester
- Zen Courses in Coventry
- Zen Courses in Bradford
- Zen Courses in Cardiff
- Zen Courses in Belfast
- Zen Courses in Nottingham

