- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
3061 TEC courses delivered Live Online
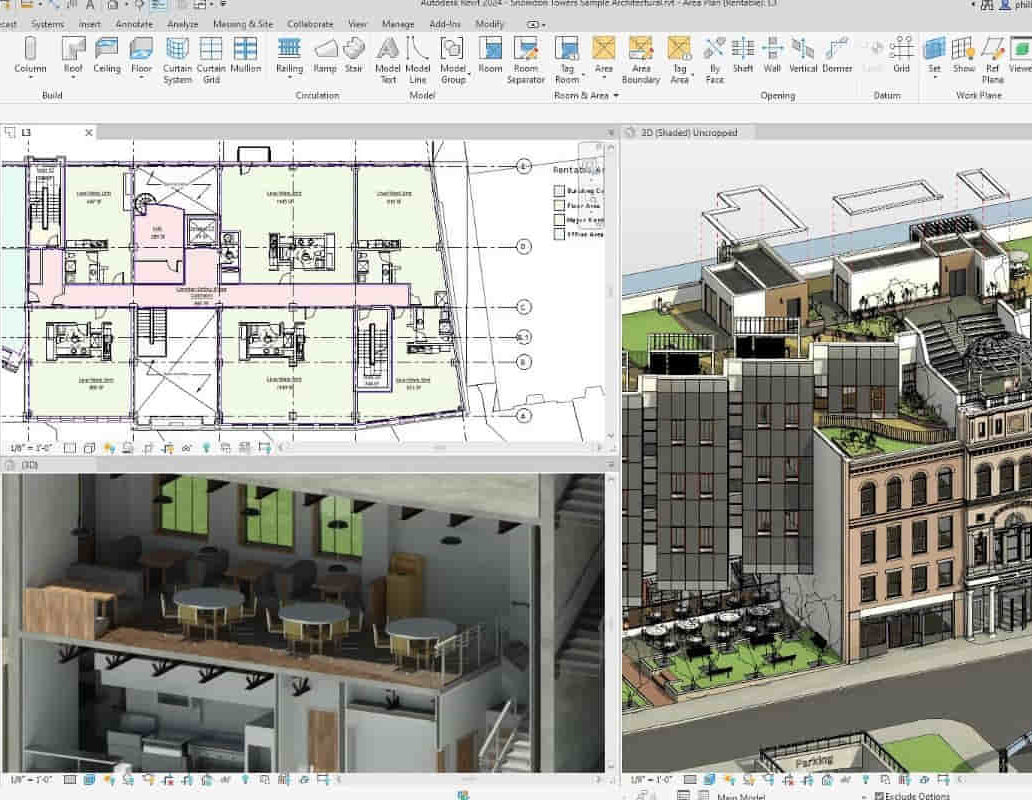
Revit V-Ray 3ds Max Visualization Training Course
By ATL Autocad Training London
Why Learn Revit V-Ray 3ds Max Visualization Training Course? Revit is perfect for architecture, and 3ds Max, with V-Ray rendering, offers super results. Autodesk Revit Interoperability integrates Revit and 3ds Max seamlessly, combining metadata, materials, and lighting. Architects and designers using 3ds Max tools for architectural designs. Check our Website Booking: 1-on-1 Duration: You can split these 40 hours over as many days, Mon to Sat between 9 am to 7 pm, or call 02077202581 to book In-person or Live Online Module 1: Revit Fundamentals (16 hours) Introduction to Revit: Acquaint yourself with Revit's essential concepts and tools. Interface and Workspace: Navigate Revit's interface and workspace seamlessly. Building Information Modeling (BIM) Workflow: Grasp the fundamental BIM workflow principles for effective modeling. Basic Building Model Creation: Learn to craft a foundational building model using Revit. Basic Modeling Techniques: Master elements like walls, doors, windows, floors, roofs, stairs, and railings. Advanced Revit Modeling: Dive into advanced topics, including family creation and working with intricate geometries. Annotation and Documentation: Enhance your projects with text, dimensions, schedules, and efficient printing/exporting methods. Module 2: 3ds Max Mastery (16 hours) Introduction to 3ds Max: Familiarize yourself with the core aspects and interface of 3ds Max. 3D Modeling Workflow: Understand the intricacies of creating basic 3D models in 3ds Max. Basic 3D Modeling Techniques: Work with primitive shapes, splines, and create complex 3D models. Materials and Textures: Explore the material editor, apply materials, and craft realistic textures. Lighting and Cameras: Learn to illuminate scenes, utilize the daylight system, and optimize camera angles. Module 3: V-Ray Rendering Proficiency (8 hours) Introduction to V-Ray: Grasp the fundamentals of V-Ray, including its interface and workflow. V-Ray Materials and Textures: Understand the intricacies of V-Ray materials and textures for lifelike renderings. Basic Rendering Techniques: Dive into essential rendering techniques, including lighting adjustments and global illumination. Advanced Rendering Methods: Explore advanced techniques to elevate the quality of your renders. Gain expertise in Revit, 3ds Max, and V-Ray through our comprehensive training, mastering the core concepts and advanced techniques necessary for stunning architectural visualizations. Upon completing the course, you will: Master Architectural Modeling: Proficiently create intricate architectural models using Revit and 3ds Max. Visualize Realistically: Utilize advanced rendering techniques in V-Ray for lifelike visualizations with realistic lighting and materials. Efficient Documentation: Learn annotation and documentation skills for clear and effective project communication. Enhance Collaboration: Understand file interoperability for seamless collaboration with diverse software platforms. Build a Professional Portfolio: Create a diverse portfolio showcasing your skills in architectural visualization. Career Opportunities: Upon completion, you can pursue roles such as: Architectural Visualizer 3D Modeler Render Artist BIM Specialist Freelancer/Consultant Architectural Technician Visualization Trainer Mastering these tools opens doors to versatile career opportunities in architectural visualization. Download Revit Download 3ds max Download Vray Revit and 3dsMax V-Ray Visualization Training: Master architectural visualization with our intensive course blending Revit, 3dsMax, and V-Ray. Dive into modeling, materials, lighting, and rendering for photorealistic results. Course Highlights: Comprehensive Learning: Cover fundamental and advanced techniques in Revit and 3dsMax, enhancing your visualization skills. V-Ray Expertise: Explore advanced V-Ray rendering techniques, from global illumination to realistic material creation. Real-World Projects: Work on industry-relevant projects, building a portfolio of professional-grade visualizations. Flexible Learning: Choose in-person or live online sessions for interactive instruction and engaging discussions. Lesson Recordings: Access session recordings to review concepts, reinforcing your learning at your own pace. Lifetime Support: Enjoy lifetime email support for ongoing guidance from experienced instructors, ensuring your success beyond the course. Enroll now to transform your architectural designs into captivating visualizations, impressing clients with your skills and creativity. Join our training for a comprehensive learning experience, enhancing your expertise in Revit, 3dsMax, and V-Ray. Revit | 3ds max | V-Ray Course Benefits: Comprehensive Skill Set: Master Revit, 3dsMax, and V-Ray for exceptional architectural visualization, from basic modeling to advanced rendering. Realistic Rendering: Harness V-Ray's capabilities for lifelike visuals, covering global illumination, materials, and textures. Practical Experience: Work on industry-relevant projects, building a professional portfolio with hands-on exercises. Flexible Learning: Choose in-person or live online sessions for interactive instruction and discussion, tailored to your preferences. Review Resources: Access recorded class sessions to reinforce your knowledge and skills at your convenience. Lifetime Support: Enjoy lifetime email support from experienced instructors, ensuring ongoing guidance for your projects.
Certified Scrum Product Owner: In-House Training
By IIL Europe Ltd
Certified ScrumMaster®: In-House Training This course is an introduction to Scrum and the principles and tools required to be an effective Scrum Product Owner. You will come away with a good understanding of the Scrum framework and the underlying principles required to make effective decisions regarding the application of the Scrum framework to different situations. Participants successfully completing this course earn a Certified Scrum Product Owner® (CSPO®) designation. The Scrum Alliance certification includes a one-year membership with Scrum Alliance. What You Will Learn You'll learn how to: Use the principles, practices, and tools required to be an effective Scrum Product Owner Make effective decisions regarding the application of the Scrum framework to different situations, including: Setting product vision and goals Chartering the project Writing user stories and structuring your product backlog Scaling the Product Owner Estimating for forward planning Applying prioritization techniques Planning and tracking release progress Getting Started Introduction Course structure Course goals and objectives Agile Principles and Scrum Overview Process control models Incremental and iterative development Shifting the focus on product management Overview of the Scrum process Agile principles Lean principles Scrum Roles and Responsibilities Scrum roles Cross-functional teams Product Owner Responsibilities The Scrum Project Community What happens to my traditional role in Scrum? Chartering the Project Establishing a shared vision Elevator Statement Data sheets Product Vision Box Magazine Review / Press Release Product Backlog and User Stories Product uncertainty and progressive refinement User role modeling User Stories Product backlog characteristics Getting backlog items ready Slicing User Stories Using the product backlog to manage expectations Sprints Done and Scaling Done The Scrum process in detail Sustainable pace The Product Owner's role in each of the Scrum meetings Scaling the Product Owner Scaling Scrum Approaches to scaling the Product Owner Estimation for Forward Planning Why comparative estimation works Planning Poker Affinity Estimation Prioritization Techniques Additional Product Backlog Prioritization Techniques Kano Analysis Theme Screening Release Planning and Tracking Progress Velocity Release Planning Tracking release progress

AgilePM Foundation: Virtual In-House Training
By IIL Europe Ltd
AgileBA® Foundation and Practitioner: Virtual In-House Training The AgileBA® Foundation and Practitioner course takes you through a business understanding of the external and internal forces that underline the project from a business perspective, looks at modeling techniques, (As Is - To Be), and also provides an overview to project management (AgilePM) from an 'Agile' perspective. The course explains the role's relevance and involvement throughout the project.

This class is designed for people who want to Learn Salsa\Zumba or any Latin Dance rhythm private class on Zoom platform for couples or individuals to achieve a good knowledge of the Salsa, Samba, Bachata, Merengue or Latin dance steps One to One lesson, 4 minimum pack lesson booking. Taught by native instructor You can have this lesson at our premises, on Zoom, Pre-recorded or home visit, the choice is yours!!! The Program includes: 10 or 20 Lesson 1on1 lesson Walking & Leading techniques Female & Male Teachers Footwork & Upper body techniques Steps & routines names (brain method) Body posture and language Tempo & beat music technique Fully equipped dance studio Ownership of your footage work progress & Docs Music library via Spotify & Apple Music Personal online library on Google Drive or Dropbox Salsa or Latin Dance rhythm private class in London for couples or individuals to achieve a good knowledge of the salsa, samba, bachata, merengue or Latin dance steps One to One lesson, 4 minimum pack lesson book Teach by native instructor We have been training people in salsa in london for almost 20 years and still in business as salsa in west london is increasing massively.

Certified Associate in Project Management (CAPM) Exam Prep: In-House Training
By IIL Europe Ltd
Certified Associate in Project Management (CAPM)® Exam Prep: In-House Training: In-House Training This course gives you the knowledge you need to pass the exam and covers CAPM®-critical information on project management theory, principles, techniques, and methods Are you planning on taking the CAPM® examination? This course gives you the knowledge you need to pass the exam and covers CAPM®-critical information on project management theory, principles, techniques, and methods. You'll also have an opportunity for practical applications and time to review the kinds of questions you'll find in the CAPM® Exam. What you Will Learn Apply for the CAPM® Examination Develop a personal exam preparation plan Describe the structure, intent, and framework principles of the current edition of the PMBOK® Guide Explain the PMBOK® Guide Knowledge Areas, as well as their inter-relationships with the each other and the Process Groups Getting Started Program orientation The CAPM® certification process Certified Associate in Project Management (CAPM®) Examination Content Outline CAPM® eligibility requirements Code of Ethics and Professional Conduct Application options Foundation Concepts Skills and qualities of a project manager Project management terminology and definitions Relationship of project, program, portfolio, and operations management Project lifecycle approaches Project Integration Management Review Project Integration Management Knowledge Area Develop Project Charter Develop Project Management Plan Direct and Manage Project Work Manage Project Knowledge Monitoring and Controlling Perform Integrated Change Control Close Project or Phase Project Stakeholder Management Review Project Stakeholder Management Knowledge Area Identify Stakeholders Plan Stakeholder Engagement Manage Stakeholder Engagement Monitor Stakeholder Engagement Project Scope Management Review Project Scope Management Knowledge Area Plan Scope Management Collect Requirements Define Scope Create WBS Validate Scope Control Scope Project Schedule Management Review Project Schedule Management Knowledge Area Plan Schedule Management Define Activities Sequence Activities Estimate Activity Durations Develop Schedule Control Schedule Project Cost Management Review Project Cost Management Knowledge Area Plan Cost Management Estimate Costs Determine Budget Control Schedule Project Resource Management Review Project Resource Management Knowledge Area Plan Resource Management Estimate Activity Resources Acquire Resources Develop Team Manage Team Control Resources Project Quality Management Review Project Quality Management Knowledge Area Plan Quality Management Manage Quality Control Quality Project Risk Management Review Project Risk Management Knowledge Area Plan Risk Management Identify Risks Perform Qualitative Risk Analysis Perform Quantitative Risk Analysis Plan Risk Responses Implement Risk Responses Monitor Risks Project Communications Management Review Project Communications Management Knowledge Area Plan Communications Management Manage Communications Monitor Communications Project Procurement Management Review Project Procurement Management Knowledge Area Plan Procurement Management Conduct Procurements Control Procurements Summary and Next Steps Program Review Mock CAPM® Exam Getting Prepared for the CAPM® Exam After the CAPM® Exam

Apple Device Support Essentials
By Influential Training
Apple course, macOS Support Essentials, ACSP, IT Support,

Collaborative Selling Activator
By Fred Copestake
Designed for professional B2B salespeople to develop a modern approach to winning business

Collaborative Selling Academy
By Fred Copestake
Designed for professional B2B salespeople to develop a modern approach to winning business

macOS Support Essentials 14 Sonoma
By Influential Training
Support Essentials course, macOS Support Essentials,

The Magic of Mentoring
By Mpi Learning - Professional Learning And Development Provider
In this course you will understand what mentoring is about, understand your roles in the process. Gain self-insight into your own interpersonal style and way of relating to others that may affect mentoring interactions.
