- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
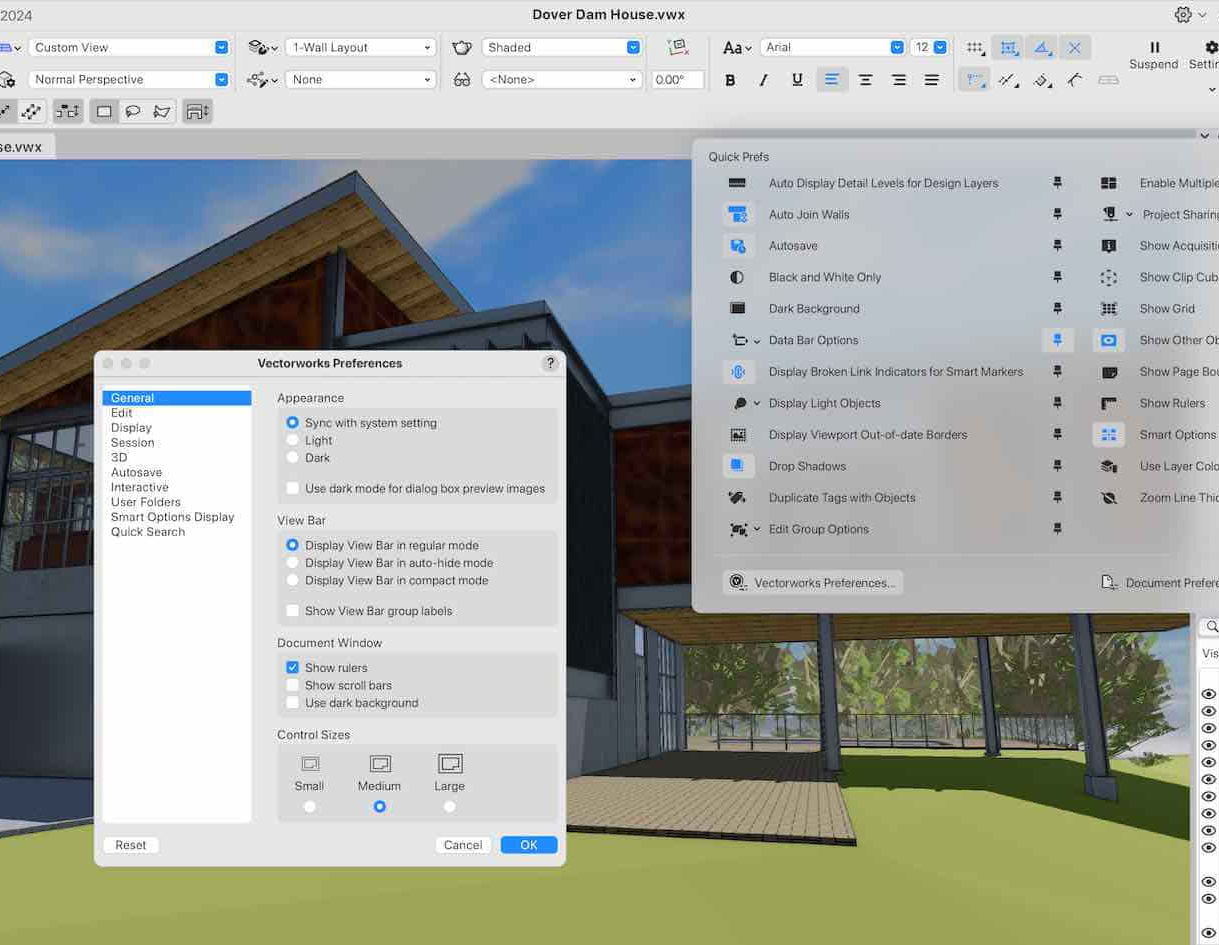
Vectorworks 2D Basics Level Training Course
By ATL Autocad Training London
Why Vectorworks 2D Basics Level Training Course? Vectorworks excels in 2D design, delivering exceptional results and serving as a robust 2D design tool. The Fundamentals course provides customizable tools for precise 2D drawings, while VectorWorks Architect streamlines the process with user-friendly, easy-to-learn tools. Click here for more info: Website Course Details: 10 hrs, Flexible 1-on-1 sessions, in-person or online. 9 am and 7 pm (Mon-Sat).10 hours, split as needed for your schedule. Module 1: Understanding CAD and Vectorworks The Role of CAD in Design Introduction to Vectorworks Software Module 2: Setting Up Your Workspace Workspace Configuration Basic Options and Preferences Module 3: Foundational Drawing Skills Drawing Techniques Selecting Objects Module 4: Advanced Object Editing Combining Shapes Mirroring Objects Rotating Objects Grouping Objects Module 5: Precision Drawing and Scaling Working with Scale Setting Preferences Saving Templates Module 6: Accurate Object Placement Drawing Precision Moving Objects Precisely Module 7: Advanced Editing Techniques Using Fillet Employing Offset Module 8: Introduction to Object Attributes and Groups Basic Attributes Editing Object Groups Module 9: Duplicating Objects Linear Duplicates Rectangular Duplicates Circular Duplicates Module 10: Creating Room Plans Designing Walls Incorporating Windows and Doors Module 11: Room Plan Development Room Plan Drawing Methods Module 12: Utilizing Additional Attributes Hatch Patterns Gradients Image Fills Module 13: Drawing Elevations Elevation Techniques Effective Methods Module 14: Importing Images for Graphics Graphic Illustration Image Integration Module 15: Symbols and Resource Management Creating Symbols Introduction to Resource Browser Module 16: Organizing Drawings with Design Layers Design Layer Usage Module 17: Labeling Drawings and Title Blocks Drawing Labels Title Block Text Module 18: Plotting and Printing User Interface and Terminology Printing Techniques Module 19: Creating Drawing Sheets A1, A2, and A3 Sheets Module 20: Utilizing Viewports Multiple Views Module 21: Professional Model Presentation Paper Space Presentation Converting to PDFs Module 22: Managing Files and Projects Module 23: Displaying Objects and Terminology Module 24: Objects and Data Management Module 25: Precise Object Placement Object Snaps Quick Select Module 26: Dividing and Measuring Objects Module 27: Dimensioning and Annotation Module 28: Working with Text Module 29: Custom Tool Palettes Module 30: Organizing Tool Palettes Module 31: Effective Tool Palette Usage Module 32: Standard Views and Drawing Techniques Module 33: Drawing Curves Arcs, Donuts, and Ellipses Module 34: Real-World Units and Measurements Module 35: Object Manipulation Changing Object Angles Module 36: File Management Saving, Exiting, and Opening Projects Module 37: Creating Mirror Images Module 38: Introduction to 3D Modeling Creating Extrusions Basic 3D Concepts Outcomes and Vectorworks Jobs: Vectorworks Proficiency: Gain expertise in using Vectorworks software for design tasks. 2D Design Skills: Create accurate 2D drawings and architectural plans. Advanced Editing: Efficiently manipulate and edit objects in your designs. Precision Drawing: Develop skills for precise scaling and drawing. These skills open doors to careers in design, architecture, engineering, entertainment, and more. Potential Jobs: Architectural Drafter Interior Designer Landscape Designer AD Technician Graphic Designer Construction Estimator Product Designer Set Designer Event Planner Urban Planner Vectorworks 2D Training Course Our Vectorworks training is thoughtfully designed to educate and inspire designers at every skill level. Whether you're just starting or a seasoned pro, our courses will furnish you with fresh skills, streamline your workflows, and unleash the full potential of your Vectorworks software. Online Training Choices Tailored Online Sessions: Customized training sessions tailored to your specific requirements and skill level. Virtual Classroom: Participate in interactive virtual classes from the convenience of your workspace. Getting Started Guides: Comprehensive guides to assist you in navigating the fundamentals of Vectorworks. In-Person Training Opportunities Customized On-Site Sessions: Hands-on training delivered directly to your office or at regional events. One-to-One: Engage in interactive learning at our training locations. Download Vectorworks https://www.vectorworks.net Personalized One-on-One Training: Get individualized attention and customized instruction. Flexible Scheduling: Choose your preferred training time and day to suit your schedule. Post-Course Assistance: Access free online support after course completion. Comprehensive Learning Materials: Receive PDF notes and handouts to enhance your learning. Certificate of Completion: Earn a recognition certificate upon successfully finishing the course. Affordable Rates: Enjoy cost-effective training rates. Software Setup Assistance: Receive help setting up the software on your computer. Referral Benefits: Recommend a friend and receive discounts on future courses. Group Training Discounts: Special discounts available for group training sessions. Convenient Availability: Access training sessions from Monday to Sunday, with extended hours. Tailored Training: Receive customized, bespoke training tailored to your specific requirements.
Leadership: Self-doubts into Self-Beliefs
By Mpi Learning - Professional Learning And Development Provider
Being a leader can take you into vulnerable places - the unfamiliar, high expectations and high visibility, with everyone looking to you for guidance in the big moments. It is natural for doubts to creep in, including the echoes of past knock-backs, put-downs, pressures, stereotyping and internalised oppression.

AutoCAD 3D Basics-Advanced Training
By London Design Training Courses
Why Learn AutoCAD 3D Basics-Advanced Training Course? Course Link Learn 3D modeling, materials, lighting, and rendering, and parametric models. Learn real-world Architecture, products, mechanics. Enroll for in-person or online sessions to master AutoCAD 3D. Duration: 16 hrs Method: 1-on-1 Schedule: Tailor your own schedule by pre-booking a convenient hour of your choice, available from Mon to Sat between 9 am and 7 pm. "Dial 02077202581 or WhatsApp 07970325184 to reserve your preferred dates and times. AutoCAD 3D Basics-Advanced Level 1-2-1 Training Course: Comprehensive coverage from basics to advanced techniques. Suitable for beginners and those advancing their 3D modeling skills. Personalized attention in a private learning environment. Experienced instructors with expertise in 3D modeling. Hands-on practice for confident 3D modeling. Flexible scheduling and lifetime email support. Certificate upon course completion for career advancement AutoCAD 3D Basics-Advanced Level 1-2-1 Training Course: Comprehensive coverage from basics to advanced techniques. Suitable for beginners and those advancing their 3D modeling skills. Personalized attention in a private learning environment. Experienced instructors with expertise in 3D modeling. Hands-on practice for confident 3D modeling. Flexible scheduling and lifetime email support. Certificate upon course completion for career advancement AutoCAD 3D Basics to Advanced Level Training Course Outline (10 hours): Session 1: Introduction to 3D Modeling (2 hours) Understanding 3D modeling concepts Working in the 3D Modeling Workspace Drawing 3D objects with Solid tools Extruding 2D polylines to create 3D objects Session 2: Advanced 3D Features and UCS (2 hours) Setting up AutoCAD for advanced 3D modeling Mastering the User Coordinate System (UCS) Using viewports to aid in 3D drawing Efficient modeling with Array tools Session 3: Rendering and Visualization (2 hours) Testing rendering techniques Creating a quick study rendering Simulating natural lighting and the sun Enhancing 3D models with materials and lights Session 4: Editing 3D Solids and Mesh Modeling (2 hours) Understanding solid modeling principles Creating and editing basic solid forms Editing 3D solids and streamlining 2D drawing process Exploring 3D mesh modeling and editing The AutoCAD 3D training course provides a comprehensive understanding of 3D modeling, visualization, and rendering. Participants will learn to create complex 3D models, render realistic visuals, and efficiently customize the AutoCAD interface for improved productivity. Master 3D Modeling: From basics to advanced techniques. Realistic Rendering: Achieve lifelike visualizations. Professional Presentations: Dynamic walkthroughs and showcases. Enhanced Career Opportunities: Stronger prospects in design fields. Personalized Learning: One-to-one attention for focused training. Practical Hands-On Practice: Building confidence in 3D modeling. Lifetime Email Support: Ongoing assistance beyond the course. Certification: Proof of proficiency in AutoCAD 3D modeling.
Master Big Data Ingestion and Analytics with Flume, Sqoop, Hive and Spark
By Packt
A complete course on Sqoop, Flume, and Hive: Ideal for achieving CCA175 and Hortonworks Spark Certification

Level 5 Diploma in Education & Training Online | L5 Diploma
By Study Plex
Highlights of the Course Course Type: Self-Paced Online Learning Guided Learning Hours: 360 Teaching Practice Hours: 100 Accreditation: NCFE Qualification: Nationally Recognised Qualification Study Materials: High-Quality E-Learning Study Materials Certificate: Certificate upon passing the official exam Access: Lifetime Access Tutor Support- Personalised feedback on your all assignments Customer Support: 24/7 live chat available What you will learn from this course? Upon successful completion of this Level 5 Diploma in Education and Training, you will be able to: Include learning strategies, concepts, and models in the design of inclusive classroom instruction and learning Create and maintain a safe, pleasant teaching and learning environment by utilising behaviour control techniques Deliver inclusive instruction and learning utilizing communication and learning theories, ideas, and models Use assessment theories, models, and principles to illustrate how to evaluate learning in training and education Utilise theories and techniques for reflection and assessment, and evaluate one's own approach to planning, executing, and evaluating inclusive teaching and learning Learn how to conduct an initial and diagnostic assessment to determine an individual's earning goals as well as the relationships, roles, and processes that are part of education and training Establish and maintain a safe environment for teaching and learning, as well as how to plan for and carry out inclusive teaching Use theories and models of curriculum creation in your area of expertise and how to use those theories to assess your own methods Recognise professionalism and the influence it has on education and training, as well as the impact of accountability to stakeholders and outside organisations Understand how education and training are governed by policy and support your organisation's attempts to improve quality Level 5 Diploma in Education & Training Online | L5 Diploma This Level 5 Diploma in Education and Training is accredited by NCFE and regulated by Ofqual, making it a nationally recognised credential that will improve your CV and set you apart from the competition. This is the ideal approach for teachers who have already finished levels 3 and 4 or aiming to achieve Qualified Teacher Status (QTLS). This course will help you gain advanced knowledge and skills to lead learning sessions, organise lesson plans, and create teaching and learning strategies. Towards the end of this course, you'll acquire an extensive range of teaching abilities and expertise you need to establish a successful career in the education sector, as well as gain the status to start working as a teacher. Who is this Course for? The following individuals may benefit from this Level 5 Diploma in Education and Training: all types of trainers aspirant teachers teachers of all levels teacher trainers teachers wishing to achieve Qualified Teacher Status (QTLS) teachers wishing to improve their instructional abilities teachers wishing to enhance their employment prospects Whether you are a complete beginner or an aspiring professional, this course will provide you with the necessary skills and professional competence, and open your doors to a wide number of professions within your chosen sector. Eligibility Requirements This Level 5 Diploma in Education and Training has no academic prerequisites and is open to students from all academic disciplines. Assessment Procedure Upon successful completion of this Level 5 Diploma in Education and Training, you will be evaluated by a series of assignments that the instructors of the course will internally construct and grade. This strategy will give students more confidence to ace the exam because it precisely represents the content taught in class and fits the teaching methodology being used. "Pass" represents the qualification's overall grade Each item included in the qualification framework must be completed by the student For a student to pass a unit, they must demonstrate their understanding of each unit by achieving all of the listed learning outcomes and meeting all of the evaluation criteria Assignment Submission For each portion of the diploma, you are required to submit a number of assignments each weighing about 1000-2000 words. Product evidence requirements, such as lesson plans, teaching resources, and other pertinent paperwork, must also be accompanied by some of these assignments. Assignments should incorporate theory and establish connections to actual workplace or classroom circumstances in order to convey pertinent arguments. These assignments will aid in your exploration and application of the entire teaching and learning cycle. If more work is needed, your instructor will offer feedback and the chance to resubmit assignments. Practical Teaching Assessment There must be a minimum of 8 observations, each lasting at least 8 hours. This excludes any observed practice that was completed as a component of the Level 3 Award in Education and Training. A minimum of 30 minutes must pass between each observation. This witness evidence must be given by a teacher who possesses the necessary qualifications. In extraordinary circumstances, we may also set up a video link with your tutor to finish these observations. The eight observations must relate to the following essential units: Education and training teaching, learning, and evaluation (level 4) Enhancing education and training through teaching, learning, and assessment (level 5) No previous experience is required to enrol into this course. However, you must complete 100 hours of in-class teaching experience throughout the course of the program to receive your completion certificate. Your practical teaching demonstration will need to be observed by an expert witness who has a level 5 diploma in education and training. For an extra charge of £250 + VAT for 8 hours, if you'd prefer, we can assist you by providing an expert witness. Advance Your Career This Level 5 Diploma in Education and Training will provide you with significant opportunities to enter the relevant job market and select your desired career path. Additionally, by showcasing these skills on your resume, you will be able to develop your career, face more competitors in your chosen sector, and increase your level of competition. This course will also allow you to acquire the required skills to work towards achieving the Qualified Teacher Status and progress to Bachelor of Arts (BA) or Bachelor of Science (BSc). If you are looking for a Level 4 Certificate in Education and Training, enrol into our affordable and highly informative course, which will open your door towards a wide range of opportunities within your chosen sector. This Level 5 Diploma in Education and Training is accredited by NCFE and regulated by Ofqual. The National Council for Educational Awarding (NCFE) is a national educational awarding body that is well-known and respected throughout the world, which will improve your prospects of finding employment and showcase your professional growth. Course Curriculum Course Overview Course Overview - Level 5 Diploma in Education and Training 00:00:00 Sample Useful Documents Observation Recording Requirements. 00:15:00 Session Plan Pro-Forma 00:15:00 Sample Portfolio Building Record Sheets 00:15:00 Sample Graded Observation Pro-Forma 00:15:00 Lesson 1: Roles Responsibilities of Teachers in Education Learning Lesson 1: Roles Responsibilities of Teachers in Education Learning 00:45:00 Lesson 2 : Pedagogical Principles Theory and Practice Lesson 2 : Pedagogical Principles Theory and Practice 00:40:00 Lesson 3 : Functional Skills and the Minimum Core Lesson 3 : Functional Skills and the Minimum Core 00:40:00 Lesson 4 : Planning in Education and Learning Lesson 4 : Planning in Education and Learning 00:30:00 Lesson 5 : Selecting, Creating and Using Resources Lesson 5 : Selecting, Creating and Using Resources 00:30:00 Lesson 6 : Augmenting Communication through Teaching Strategies Lesson 6 : Augmenting Communication through Teaching Strategies 00:30:00 Lesson 7 : The Assessment Process Lesson 7 : The Assessment Process 00:30:00 Lesson 8 : Managing Learners Lesson 8 : Managing Learners 00:30:00 Lesson 9 : Professionalism and Continued Professional Development Lesson 9 : Professionalism and Continued Professional Development 00:25:00 Lesson 10 : Developing Designing Curriculum Lesson 10 : Developing Designing Curriculum 00:28:00 Lesson 11 : The Professional Practice Quality Management Lesson 11 : The Professional Practice Quality Management 00:26:00 Lesson 12 - Developing, Using and Organising Resources in a Specialist Area Lesson 12 - Developing, Using and Organising Resources in a Specialist Area 00:30:00 Additional Resource Additional Resource - Level 5 Diploma in Education and Training 04:00:00 Assignment - Mandatory Units Assignment 1: Developing Teaching, Learning and Assessment in Education and Training Assignment 1 - Developing Teaching, Learning and Assessment in Education and Training 00:00:00 Assignment 2: Teaching, Learning and Assessment in Education and Training Assignment 2 - Teaching, Learning and Assessment in Education and Training 00:00:00 Assignment 3: Theories, Principles and Models in Education and Training Assignment 3 - Theories, Principles and Models in Education and Training 00:00:00 Assignment 4: Wider Professional Practice and Development in Education and Training Assignment 4 - Wider Professional Practice and Development in Education and Training 00:00:00 Assignment 5: Develop Learning and Development Programmes Assignment 5 - Develop Learning and Development Programmes 00:00:00 Assignment 6: Identify the Learning Needs of Organisations Assignment 6 - Identify the Learning Needs of Organisations 00:00:00 Assignment 7: Internally Assure the Quality of Assessment Assignment 7 - Internally Assure the Quality of Assessment 00:00:00 Assignment 8: Understanding and Managing Behaviours in a Learning Environment Assignment 8 - Understanding and Managing Behaviours in a Learning Environment 00:00:00 Assignment 9: Understanding the Principles and Practices of Internally Assuring the Quality of Assessment Assignment 9 - Understanding the Principles and Practices of Internally Assuring the Quality of Assessments 00:00:00 Assignment 10: Working with Individual Learners Assignment 10 - Working with Individual Learners 00:00:00 Feedback Feedback 00:00:00

**10 FREE QLS Endorsed Certificates and Included with Lifetime Access** Business moves fast, and those who understand how to manage it wisely often lead the way. The Level 7 Diploma in Business Administration – QLS Endorsed Course is crafted for individuals who wish to sharpen their decision-making abilities, strategic thinking, and leadership approaches in today's demanding corporate environment. Through a carefully structured online programme, you will gain the theoretical expertise needed to navigate complex business challenges with confidence and skill. This diploma focuses on developing a strong foundation in key areas such as organisational management, strategic planning, resource allocation, and communication techniques. Designed to fit around your schedule, the course allows you to build high-level knowledge without stepping into a lecture hall. Whether you are aiming to progress in your current role or broaden your administrative acumen, the Level 7 Diploma in Business Administration offers a solid stepping stone towards professional growth, all from the comfort of your own space. Business Administration - QLS Endorsed Bundle Includes the following Courses Course 01: Advanced Diploma in Business Administration at QLS Level 7 Course 02: Diploma in Business Law at QLS Level 5 Course 03: Diploma in Business Analyst Training at QLS Level 5 Course 04: Diploma in Financial Reporting at QLS Level 4 Course 05: Certificate in GDPR Training at QLS Level 3 Course 06: Diploma in Business and Management at QLS Level 5 Course 07: Diploma in Economics at QLS Level 5 Course 08: Diploma in Corporate Governance at QLS Level 5 Course 09: Award in Business Finance at QLS Level 2 Course 10: Certificate in Office Admin and Organisation Skills at QLS Level 3 Learning Outcomes Analyse complex business scenarios for strategic decision-making. Demonstrate proficiency in business law applications. Execute effective financial reporting strategies. Implement GDPR compliance measures in business operations. Apply advanced business and management principles. Evaluate economic trends and their impact on organisations. Implement corporate governance practices for ethical business conduct. Demonstrate foundational knowledge in business finance. Efficiently manage office administration and organisational tasks. Cultivate critical thinking for effective problem-solving. Key Features 10 FREE QLS Endorsed Certificate Fully online, interactive course Self-paced learning and laptop, tablet, smartphone-friendly 24/7 Learning Assistance Discounts on bulk purchases In the dynamic landscape of commerce, every decision matters. Picture yourself not just making decisions but sculpting the future of your organisation with precision. Our comprehensive bundle is not just a set of courses; it's a roadmap to mastering the intricate dance of strategy, leadership, and administration. Seamlessly progress from foundational principles to advanced strategic insights, mastering business administration at various QLS levels. Seize this opportunity to equip yourself with the skills that set the trailblazers apart. Your journey towards business excellence begins here - where theory meets practice and success is not just a goal; it's a destination. Learn the key aspects of business, from honing legal acumen in business law to becoming a proficient business analyst. Dive into financial reporting, grasp GDPR essentials, and refine your skills in business and management. Elevate your understanding of economics and corporate governance while gaining essential business finance insights. Enhance organisational proficiency with a focus on office administration. This bundle provides a holistic approach, empowering you with a well-rounded skill set for the dynamic world of business. Certificate Once you've successfully completed your course, you will immediately be sent a CPD Accredited PDF certificate. Also, you can have your printed certificate delivered by post (shipping cost £3.99). After successfully completing the assignment, learners will be able to order FREE QLS Endorsed certificate for Each Courses. CPD 55 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Aspiring business professionals seeking comprehensive expertise. Individuals aiming for a versatile skill set in business administration. Professionals desiring legal insights in the business context. Those aspiring to excel in business analysis and reporting. Finance enthusiasts looking to specialise in financial reporting. Individuals keen on understanding GDPR implications in business. Management aspirants aiming for strategic leadership roles. Office administrators seeking to enhance organisational skills. Career path Business Analyst Corporate Governance Officer Financial Analyst Business Manager Legal Consultant Economic Analyst Certificates Digital certificate Digital certificate - Included Hard copy certificate Hard copy certificate - Included Hardcopy Certificate (UK Delivery): For those who wish to have a physical token of their achievement, we offer a high-quality, printed certificate. This hardcopy certificate is also provided free of charge. However, please note that delivery fees apply. If your shipping address is within the United Kingdom, the delivery fee will be only £3.99. Hardcopy Certificate (International Delivery): For all international addresses outside of the United Kingdom, the delivery fee for a hardcopy certificate will be only £10. QLS Endorsed Certificate Hard copy certificate - Included

CMI Level 3 Award in Principles of Management and Leadership
By Karen Blake Coaching
Unlock your leadership potential with the CMI Level 3 Award in Principles of Management and Leadership on Cademy. This globally recognised qualification equips emerging leaders to set and monitor goals, provide direction, and achieve clear outcomes. Elevate your career and leadership skills today. Enrol on Cademy to take the next step! #LeadershipDevelopment #CMIQualification #ManagementSkills

M.D.D PRIVATE OCCUPATIONAL THERAPY LONDON PACKAGE (SELF IMPROVEMENT)
4.9(27)By Miss Date Doctor Dating Coach London, Couples Therapy
Introducing Private Occupational Therapy London Package: Personalized Support for Your Unique Needs Are you looking for private occupational therapy services in London that prioritize your individual needs and goals? Miss Date Doctor’s Private Occupational Therapy London Package offers you dedicated one-on-one support and personalized interventions to help you overcome challenges and improve your quality of life. Private occupational therapy provides a focused and tailored approach, ensuring that you receive the highest level of care and attention. Our experienced occupational therapists in London are committed to understanding your specific needs and providing effective solutions to enhance your daily functioning and well-being. Here’s how the Private Occupational Therapy London Package can support you: Personalized Assessment: Our private occupational therapists will conduct a thorough and individualized assessment to gain a comprehensive understanding of your unique challenges, strengths, and goals. Tailored Treatment Plan: Based on the assessment, we’ll develop a customized treatment plan designed to address your specific needs and aspirations. This plan will focus on enhancing your functional abilities and promoting independence. Focused Attention: Private occupational therapy sessions offer you dedicated one-on-one attention, ensuring that you receive the most effective and personalized interventions. Individual Progress: Our therapists will closely monitor your progress throughout the therapy process, making adjustments to the treatment plan as needed to maximize your outcomes. Convenient Scheduling: Private occupational therapy allows for flexible scheduling options to accommodate your busy lifestyle and ensure that you have access to the support you need when it’s most convenient for you. Personal Empowerment: We believe in empowering you to take an active role in your therapy journey. Our therapists will collaborate with you, providing guidance and support as you work towards your goals. Confidentiality and Privacy: Private occupational therapy sessions offer a confidential and private environment where you can freely discuss your challenges and experiences. The Private Occupational Therapy London Package at Miss Date Doctor is committed to providing you with the highest level of personalized care and support. Our expert occupational therapists will work closely with you to help you overcome obstacles, regain independence, and enhance your daily living skills. Invest in your well-being and experience the benefits of private occupational therapy. Take the first step towards a more fulfilling and empowered life with the Private Occupational Therapy London Package. Let our skilled therapists guide you towards greater independence, improved functionality, and a more enriched quality of life. 3 x1 hour https://relationshipsmdd.com/product/private-occupational-therapy-london-package/

M.D.D M.D.D COUPLES DRAMA PACKAGE (COUPLES)
4.9(27)By Miss Date Doctor Dating Coach London, Couples Therapy
Just had an argument with your girlfriend or boyfriend want to sort it out? Need assistance? An M.D.D date coach will call both of you once a day for 30minutes for one week and mediate on your behalf and do a session together at the end of the week to help you both see the other person’s point of view. (This package is only for couples who have a problem and both parties want to resolve the issue) https://relationshipsmdd.com/product/m-d-d-couples-drama-package/

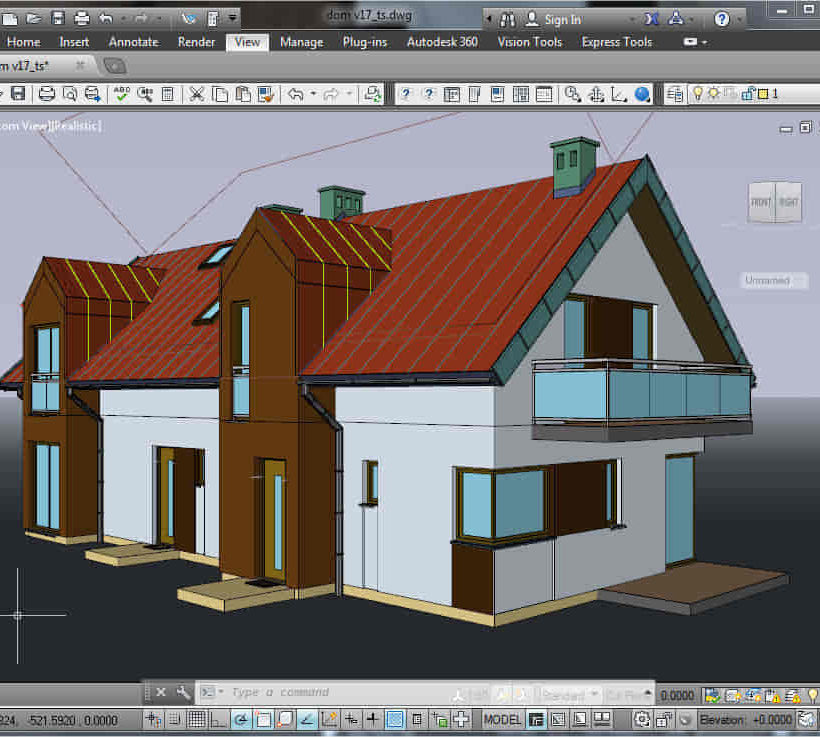
Sketchup Courses London 1 on 1
By Real Animation Works
SketchUp Course With Layout and V-Ray
