- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
10882 Professional Development courses in Wednesfield delivered On Demand
Overview Gain ultimate child safeguarding and risk management skills to become a qualified professional, by taking the all-inclusive Child Safeguarding Officer Training Diploma course.The exclusive Child Safeguarding Officer Training Diploma course covers all the safeguarding fundamentals, including UK child safeguarding laws and regulations, risk assessments, record keeping, and strategies. By attending the course, you'll develop effective communication skills and the personal leadership skills to perform your safeguarding responsibilities properly. You'll gain a solid idea on child psychology that will help you deal with any safeguarding crisis thoroughly. Ensure your professional development by enrolling today! How will I get my certificate? You may have to take a quiz or a written test online during or after the course. After successfully completing the course, you will be eligible for the certificate. Who is this course for? There is no experience or previous qualifications required for enrolment on this Child Safeguarding Officer Training Diploma. It is available to all students, of all academic backgrounds. Requirements Our Child Safeguarding Officer Training Diploma is fully compatible with PC's, Mac's, Laptop, Tablet and Smartphone devices. This course has been designed to be fully compatible on tablets and smartphones so you can access your course on wifi, 3G or 4G.There is no time limit for completing this course, it can be studied in your own time at your own pace. Career path Having these various qualifications will increase the value in your CV and open you up to multiple sectors such as Business & Management , Admin, Accountancy & Finance, Secretarial & PA, Teaching & Mentoring etc. Course Curriculum 8 sections • 8 lectures • 03:16:00 total length •Introduction to Safeguarding: 00:21:00 •Laws and Guidance: 00:41:00 •Child Abuse: 00:46:00 •Child Sexual Exploitation: 00:34:00 •Responding to Disclosure and Reporting: 00:25:00 •Risks and Risk Assessment: 00:14:00 •: 00:00:00 •Record Keeping: 00:15:00

Overview Enrol in our Quality Management today and build the necessary skills, knowledge and experience to transform your career. The Quality Management could enhance your continuing professional development thus propelling you more towards your dream job. This Quality Management could be your key, if you are interested in a long term career in the field of Quality Management. This Quality Management course consists of a number of easy to digest, in-depth modules which are designed to provide you with detailed knowledge on Quality Management. This Quality Management aims to accompany you through your journey to help you become a master of Quality Management. Learn through a mixture of interactive lessons and online study materials. How will I get my certificate? You may have to take a quiz or a written test online during or after the course. After successfully completing the course, you will be eligible for the certificate. Who is this course for? There is no experience or previous qualifications required for enrolment on this Quality Management. It is available to all students, of all academic backgrounds. Requirements Our Quality Management is fully compatible with PC's, Mac's, Laptop, Tablet and Smartphone devices. This course has been designed to be fully compatible on tablets and smartphones so you can access your course on wifi, 3G or 4G. There is no time limit for completing this course, it can be studied in your own time at your own pace. Career path Having these various qualifications will increase the value in your CV and open you up to multiple sectors such as Business & Management , Admin, Accountancy & Finance, Secretarial & PA, Teaching & Mentoring etc. Course Curriculum 9 sections • 9 lectures • 02:39:00 total length •Introduction to Quality Management: 00:26:00 •Total Quality Management: 00:17:00 •Quality Measurement and Improvement: 00:25:00 •Quality Control: 00:13:00 •Understanding Customer Expectations and Needs: 00:16:00 •Six Sigma: 00:21:00 •Supply Chain Management: 00:25:00 •Quality Audits: 00:16:00 •Order your Certificate: 00:00:00

Money, Banking, Monetary Policy, TAX, Accounting, Economics, Finance, Investment & AML - 20 Courses Bundle
By NextGen Learning
Get ready for an exceptional online learning experience with the Money, Banking, Monetary Policy, TAX, Accounting, Economics, Finance, Investment & AML bundle! This carefully curated collection of 20 premium courses is designed to cater to a variety of interests and disciplines. Dive into a sea of knowledge and skills, tailoring your learning journey to suit your unique aspirations. The Money, Banking, Monetary Policy, TAX, Accounting, Economics, Finance, Investment & AML is a dynamic package, blending the expertise of industry professionals with the flexibility of digital learning. It offers the perfect balance of foundational understanding and advanced insights. Whether you're looking to break into a new field or deepen your existing knowledge, the Money & Banking package has something for everyone. As part of the Money, Banking, Monetary Policy, TAX, Accounting, Economics, Finance, Investment & AML package, you will receive complimentary PDF certificates for all courses in this bundle at no extra cost. Equip yourself with the Money & Banking bundle to confidently navigate your career path or personal development journey. Enrol today and start your career growth! This Bundle Comprises the Following CPD Accredited Courses: Central Banking Monetary Policy Digital Banking Bank Teller Raise Money and Valuation for Business Banking and Finance Accounting Statements Financial Analysis UK Tax Accounting Introduction to VAT Applied Economics Anti Money Laundering & Countering of Terrorist Financing Introduction to Accounting Capital Budgeting & Investment Decision Rules Debt Management - Online Course Hacked Credit and Debit Card Recovery Course Finance Principles Financial Reporting Career Development Plan Fundamentals CV Writing and Job Searching Learn to Level Up Your Leadership Networking Skills for Personal Success Ace Your Presentations: Public Speaking Masterclass Learning Outcome: Gain comprehensive insights into multiple fields. Foster critical thinking and problem-solving skills across various disciplines. Understand industry trends and best practices through the Money & Banking Bundle. Develop practical skills applicable to real-world situations. Enhance personal and professional growth with Money & Banking Bundle. Build a strong knowledge base in your chosen course via Money & Banking Bundle. Benefit from the flexibility and convenience of online learning. With the Money & Banking package, validate your learning with a CPD certificate. Each course in this bundle holds a prestigious CPD accreditation, symbolising exceptional quality. The materials, brimming with knowledge, are regularly updated, ensuring their relevance. This bundle promises not just education but an evolving learning experience. Engage with this extraordinary collection, and prepare to enrich your personal and professional development. Embrace the future of learning with the Money, Banking, Monetary Policy, TAX, Accounting, Economics, Finance, Investment & AML, a rich anthology of 15 diverse courses. Each course in the Money & Banking bundle is handpicked by our experts to ensure a wide spectrum of learning opportunities. ThisMoney, Banking, Monetary Policy, TAX, Accounting, Economics, Finance, Investment & AML bundle will take you on a unique and enriching educational journey. The bundle encapsulates our mission to provide quality, accessible education for all. Whether you are just starting your career, looking to switch industries, or hoping to enhance your professional skill set, the Money, Banking, Monetary Policy, TAX, Accounting, Economics, Finance, Investment & AML bundle offers you the flexibility and convenience to learn at your own pace. Make the Money & Banking package your trusted companion in your lifelong learning journey. CPD 200 CPD hours / points Accredited by CPD Quality Standards Who is this course for? The Money, Banking, Monetary Policy, TAX, Accounting, Economics, Finance, Investment & AML bundle is perfect for: Lifelong learners looking to expand their knowledge and skills. Professionals seeking to enhance their career with CPD certification. Individuals wanting to explore new fields and disciplines. Anyone who values flexible, self-paced learning from the comfort of home. Career path Unleash your potential with the Money, Banking, Monetary Policy, TAX, Accounting, Economics, Finance, Investment & AML bundle. Acquire versatile skills across multiple fields, foster problem-solving abilities, and stay ahead of industry trends. Ideal for those seeking career advancement, a new professional path, or personal growth. Embrace the journey with the Money & Banking bundle package. Certificates Certificate Of Completion Digital certificate - Included Certificate Of Completion Hard copy certificate - Included You will get a complimentary Hard Copy Certificate.

The Know-How For Startup Business - 20 Courses Bundle
By NextGen Learning
Get ready for an exceptional online learning experience with the Know-How For Startup Business bundle! This carefully curated collection of 20 premium courses is designed to cater to a variety of interests and disciplines. Dive into a sea of knowledge and skills, tailoring your learning journey to suit your unique aspirations. The Know-How For Startup Business is a dynamic package, that blends the expertise of industry professionals with the flexibility of digital learning. It offers the perfect balance of foundational understanding and advanced insights. Whether you're looking to break into a new field or deepen your existing knowledge, the Know-How For Startup Business package has something for everyone. As part of the Know-How For Startup Business package, you will receive complimentary PDF certificates for all courses in this bundle at no extra cost. Equip yourself with the Know-How For Startup Business bundle to confidently navigate your career path or personal development journey. Enrol today and start your career growth! This Bundle Comprises the Following CPD Accredited Courses: Business Startup Advanced Pitching for Startups Investment Business Fundamentals For Small Startups Entrepreneurs Technical Analysis Masterclass for Trading & Investing Investment Pitching Diploma Entrepreneurial Mindset Capital Budgeting & Investment Decision Rules Anti Money Laundering (AML) Level 3 Training Fundamentals of Crypto Trading Commercial law Trading and Financing: Candlestick Pattern Stock Trading & Investing Stock Market Chart Patterns for Day Trading Penny Stock Day Trading Pattern for Consistent Profits Career Development Plan Fundamentals CV Writing and Job Searching Learn to Level Up Your Leadership Networking Skills for Personal Success Ace Your Presentations: Public Speaking Masterclass Learning Outcome: Gain comprehensive insights into multiple fields. Foster critical thinking and problem-solving skills across various disciplines. Understand industry trends and best practices through the Know-How For Startup Business Bundle. Develop practical skills applicable to real-world situations. Enhance personal and professional growth with the Know-How For Startup Business Bundle. Build a strong knowledge base in your chosen course via the Know-How For Startup Business Bundle. Benefit from the flexibility and convenience of online learning. With the Know-How For Startup Business package, validate your learning with a CPD certificate. Each course in this bundle holds a prestigious CPD accreditation, symbolising exceptional quality. The materials, brimming with knowledge, are regularly updated, ensuring their relevance. This bundle promises not just education but an evolving learning experience. Engage with this extraordinary collection, and prepare to enrich your personal and professional development. Embrace the future of learning with The Know-How For Startup Business, a rich anthology of 15 diverse courses. Each course in the Know-How For Startup Business bundle is handpicked by our experts to ensure a wide spectrum of learning opportunities. This Know-How For Startup Business bundle will take you on a unique and enriching educational journey. The bundle encapsulates our mission to provide quality, accessible education for all. Whether you are just starting your career, looking to switch industries, or hoping to enhance your professional skill set, the Know-How For Startup Business bundle offers you the flexibility and convenience to learn at your own pace. Make the Know-How For Startup Business package your trusted companion in your lifelong learning journey. CPD 210 CPD hours / points Accredited by CPD Quality Standards Who is this course for? The Know-How For Startup Business bundle is perfect for: Lifelong learners looking to expand their knowledge and skills. Professionals seeking to enhance their career with CPD certification. Individuals wanting to explore new fields and disciplines. Anyone who values flexible, self-paced learning from the comfort of home. Career path Unleash your potential with the Know-How For Startup Business bundle. Acquire versatile skills across multiple fields, foster problem-solving abilities, and stay ahead of industry trends. Ideal for those seeking career advancement, a new professional path, or personal growth. Embrace the journey with the Know-How For Startup Business bundle package. Certificates Certificate Of Completion Digital certificate - Included Certificate Of Completion Hard copy certificate - Included You will get a complimentary Hard Copy Certificate.

SUB06: Variations to Marketing Authorisations in Europe
By Zenosis
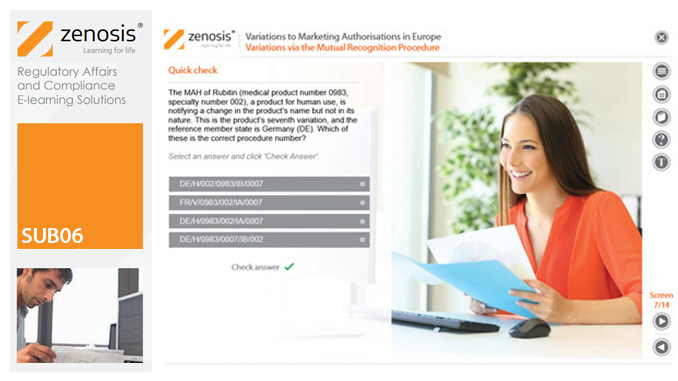
Changes to the terms of marketing authorisations for medicinal products, called variations in Europe, must be notified to or approved by the relevant regulatory authorities. Variations include changes to the composition of products, their manufacturing processes, the way they are used, or the indications for which they are authorised. Common approaches are adopted within the European Economic Area to variations to marketing authorisations approved through the Centralised, Decentralised or Mutual Recognition Procedures. Recent legislation has substantially modified the regulatory requirements and extended them to purely national authorisations by member states. This module, which is fully up to date with the new legislation, covers the classification of variations into their several types and the regulatory requirements, guidance and procedures to be followed for each type.
SUB13: How to Gain Approval to Market a Generic Drug in the USA
By Zenosis
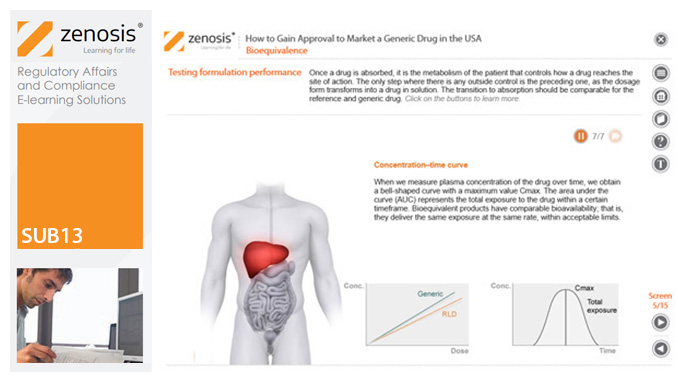
This module outlines the legislative and regulatory context for the development of generic drugs and describes the essential role of the Abbreviated New Drug Application (ANDA) in gaining marketing approval. The use of information in the ‘Orange Book’ is explained, as is the role of patent certification in the application. The importance of establishing bioequivalence between a generic and its reference product is emphasised. The module specifies the content and format requirements for an ANDA submission and describes the FDA’s review and approval process. An outline is given of the Generic Drug User Fee Amendments (GDUFA) and the law’s effects on industry players.
SUB14: The Regulatory Pathway to Licensure of Follow-on Biologics (Biosimilars) in the USA
By Zenosis
The regulation of biological medicinal products is governed by different laws from those that apply to small-molecule synthetic drugs. Producing faithful copies of therapeutic proteins is more challenging than producing generic drugs. The US legal framework for the licensure of follow-on biologics, and accompanying regulatory guidance from the Food and Drug Administration (FDA), have been established only in recent years.

SUB15: The Biologics License Application (BLA) for Marketing Approval in the USA
By Zenosis
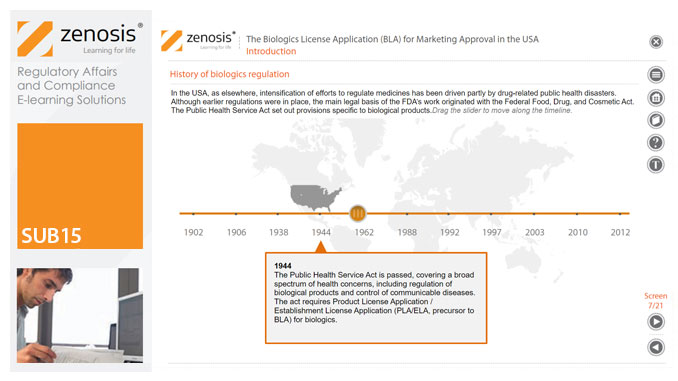
This module describes the requirements that must be met to obtain licensure of a biological product. Subjects covered include the regulatory context, the content and format of the BLA submission, the review process, and provisions for expedited development and review.
VAL06: Computer Systems Validation, Part 1: Planning
By Zenosis
In the medicines and healthcare products industries, computerised systems used in automated manufacturing or laboratory processes to which Good Manufacturing Practice requirements apply need to be validated. This module describes the planning of such validation. It follows the work of a pharmaceutical company's team as they validate the dispensary control system for a new production line.

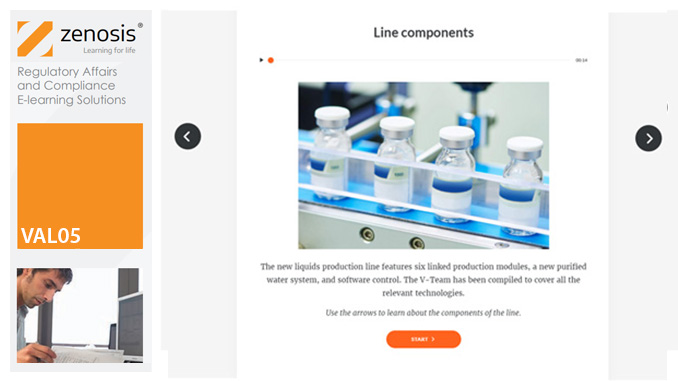
VAL05: Equipment Cleaning Validation
By Zenosis
Manufacturers of medicines and healthcare products must establish, validate and maintain an equipment cleaning programme. This is a regulatory requirement because validated cleaning procedures contribute to the assurance of product purity and safety. This module provides a comprehensive account of equipment cleaning validation requirements and procedures. It follows the work of a pharmaceutical company's validation team as they establish and validate the cleaning program for a new production line.