- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
10953 Professional Development courses in Solihull delivered On Demand
VAL01: Introduction to Validation
By Zenosis
Validation of equipment, services, systems and processes is vitally important in the medicines and healthcare products industries. Regulatory authorities require documented evidence that manufacturing processes will consistently result in products meeting predetermined quality standards. This module provides an introduction to validation and to the regulations and guidance that apply to it. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
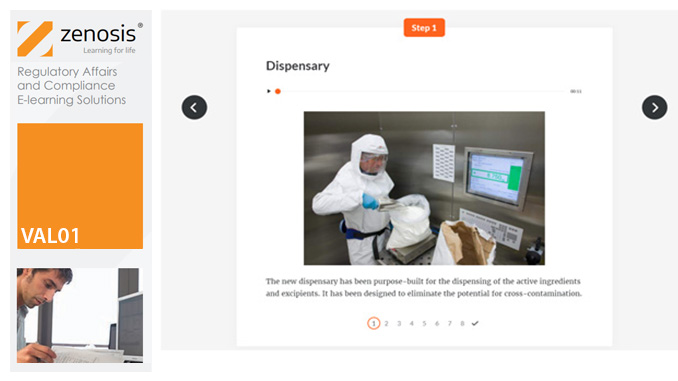
SUB13: How to Gain Approval to Market a Generic Drug in the USA
By Zenosis
This module outlines the legislative and regulatory context for the development of generic drugs and describes the essential role of the Abbreviated New Drug Application (ANDA) in gaining marketing approval. The use of information in the ‘Orange Book’ is explained, as is the role of patent certification in the application. The importance of establishing bioequivalence between a generic and its reference product is emphasised. The module specifies the content and format requirements for an ANDA submission and describes the FDA’s review and approval process. An outline is given of the Generic Drug User Fee Amendments (GDUFA) and the law’s effects on industry players.
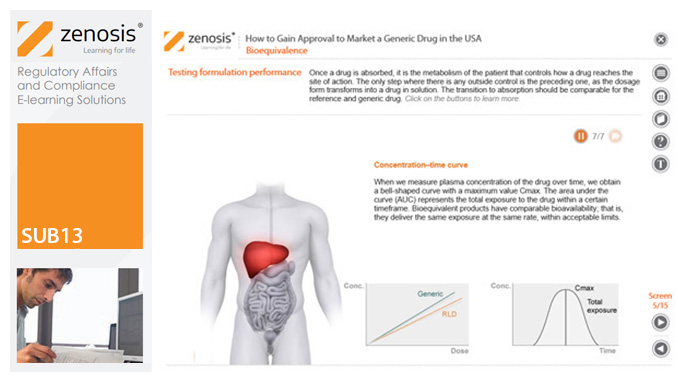
SUB06: Variations to Marketing Authorisations in Europe
By Zenosis
Changes to the terms of marketing authorisations for medicinal products, called variations in Europe, must be notified to or approved by the relevant regulatory authorities. Variations include changes to the composition of products, their manufacturing processes, the way they are used, or the indications for which they are authorised. Common approaches are adopted within the European Economic Area to variations to marketing authorisations approved through the Centralised, Decentralised or Mutual Recognition Procedures. Recent legislation has substantially modified the regulatory requirements and extended them to purely national authorisations by member states. This module, which is fully up to date with the new legislation, covers the classification of variations into their several types and the regulatory requirements, guidance and procedures to be followed for each type.
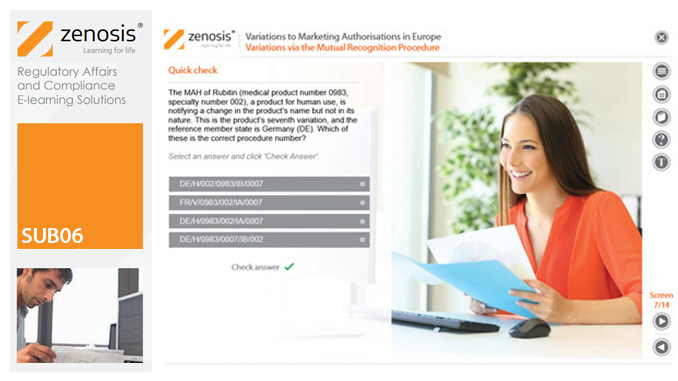
SUB14: The Regulatory Pathway to Licensure of Follow-on Biologics (Biosimilars) in the USA
By Zenosis
The regulation of biological medicinal products is governed by different laws from those that apply to small-molecule synthetic drugs. Producing faithful copies of therapeutic proteins is more challenging than producing generic drugs. The US legal framework for the licensure of follow-on biologics, and accompanying regulatory guidance from the Food and Drug Administration (FDA), have been established only in recent years.

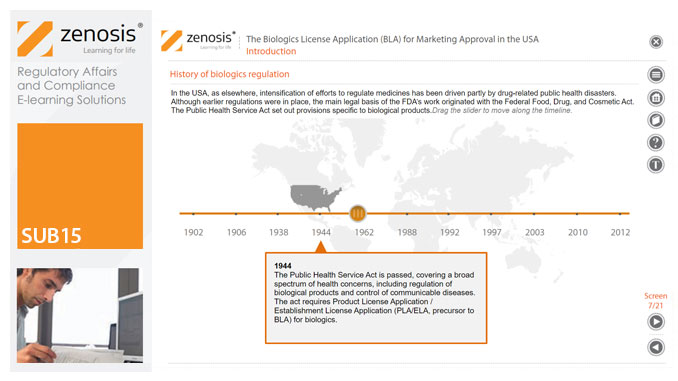
SUB15: The Biologics License Application (BLA) for Marketing Approval in the USA
By Zenosis
This module describes the requirements that must be met to obtain licensure of a biological product. Subjects covered include the regulatory context, the content and format of the BLA submission, the review process, and provisions for expedited development and review.
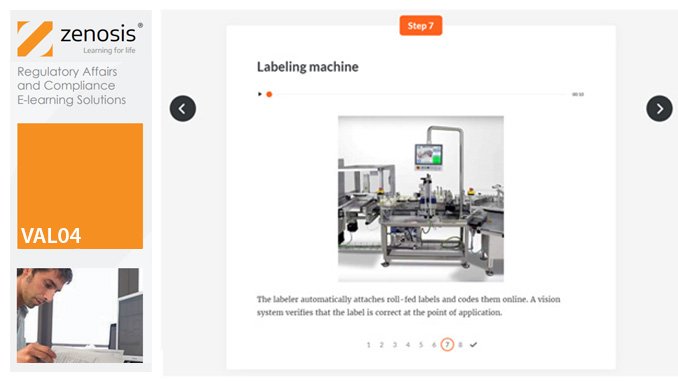
VAL04: Operational and Performance Qualification
By Zenosis
Having undergone Installation Qualification, before equipment can be used routinely in production, it needs to undergo Operational Qualification (OQ) and Performance Qualification (PQ). This module describes OQ and PQ requirements and procedures in the medicines and healthcare products industries. It follows the activities of a typical validation team as they carry out a project for a pharmaceutical company.
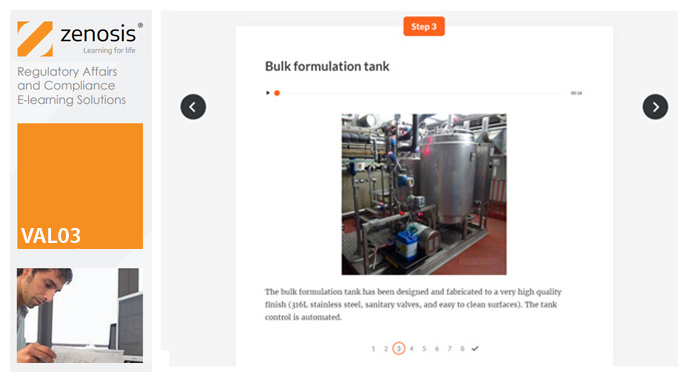
VAL03: Commissioning and Installation Qualification
By Zenosis
Before equipment can be used routinely in production, it must first be commissioned and, if necessary, undergo Installation Qualification (IQ). This module describes commissioning and IQ requirements and procedures in the medicines and healthcare products industries. It follows the activities of a typical validation team as they carry out a project for a pharmaceutical company.
ESS01: Essentials of EU and US Regulatory Affairs for Human Medicinal Products
By Zenosis
This foundation-level module is the ideal introduction for new entrants to the field of pharmaceutical regulatory affairs and compliance. It describes the principal requirements that must be satisfied to gain and maintain approval to market medicinal products in the USA and Europe. The legal framework and the roles of major players in regulation are presented. The life-cycle of a drug is outlined. The various procedures available for assessment and approval of products are described and their requirements outlined. Obligations to be fulfilled after marketing approval are discussed.

Overview This comprehensive course on 'Learning Support Assistant' will deepen your understanding on this topic. After successful completion of this course you can acquire the required skills in this sector. This 'Learning Support Assistant' comes with accredited certification which will enhance your CV and make you worthy in the job market. So enrol in this course today to fast track your career ladder. How will I get my certificate? You may have to take a quiz or a written test online during or after the course. After successfully completing the course, you will be eligible for the certificate. Who is this course for? There is no experience or previous qualifications required for enrolment on this 'Learning Support Assistant'. It is available to all students, of all academic backgrounds. Requirements Our 'Learning Support Assistant' is fully compatible with PC's, Mac's, Laptop,Tablet and Smartphone devices. This course has been designed to be fully compatible on tablets and smartphones so you can access your course on wifi, 3G or 4G. There is no time limit for completing this course, it can be studied in your own time at your own pace. Career path Having these various qualifications will increase the value in your CV and open you up to multiple sectors such as Business & Management, Admin, Accountancy & Finance, Secretarial & PA, Teaching & Mentoring etc. Course Curriculum 10 sections • 10 lectures • 03:53:00 total length •The Roles of a Learning Support Assistant (LSA): 00:26:00 •Working with Children in a School: 00:18:00 •Assessing Students: 00:12:00 •Skills of a Learning Support Assistant: 00:23:00 •Providing for All Children: 00:28:00 •Safeguarding and Wellbeing of Children and Young People: 00:22:00 •Dealing with Emergencies: 00:21:00 •Special Educational Needs (SEN): 00:38:00 •Professional Development: 00:24:00 •Time and Stress Management: 00:21:00

Lab Technician: Lab Technician Course Unlock the Power of Lab Technician: Lab Technician Course: Enrol Now! Do you want to develop your skills as a lab technician or pursue a career in it? If yes, sign up for this Lab Technician: Lab Technician Course to get the knowledge and abilities required to become more organised and productive. The goal of the Lab Technician: Lab Technician Course was to provide participants a thorough grasp of Lab technicians with a focus on legal ideas, best practices, and underlying competence. The lab technician course explains the function of the Lab Technician: Lab Technician Course as well as laboratory safety, ethics, equipment, and instruments. As a lab technician, you will be familiar with medical language, paperwork, and specimen collecting and management. This Lab Technician: Lab Technician Course explains all the responsibilities of a lab technician in pathology, professional growth job choices, and much more. Sign up for the Lab Technician: Lab Technician Course to increase your output at work. Main Course: Lab Technician Course Free Courses included with Lab Technician: Lab Technician Course Along with Level 3 Certificate in Nutrition Course you will get free Medical Terminology Course Along with Level 3 Certificate in Nutrition Course you will get free Medical secretary & receptionist Course Special Offers of this Lab Technician: Lab Technician Course: This Lab Technician: Lab Technician Course includes a FREE PDF Certificate. Lifetime access to this Lab Technician: Lab Technician Course Instant access to this Lab Technician: Lab Technician Course 24/7 Support Available to this Lab Technician: Lab Technician Course Lab Technician: Lab Technician Course With an emphasis on Lab Technician: Lab Technician Course legal concepts, Lab technician best practices and the underlying expertise, the Lab Technician: Lab Technician Course aimed to provide participants with an in-depth understanding of Lab technicians. The Lab Technician: Lab Technician Course role, laboratory safety, ethics, equipment and instruments are explained in the Lab Technician: Lab Technician Course. You will know the medical terminology, documentation, specimen collection and handling as a Lab technician. All the roles of a Lab Technician in pathology, professional development career paths and many more are explained in this lab Technician. Who is this course for? Lab Technician: Lab Technician Course Enrolling in this Lab Technician: Lab Technician Course will enhance your resume and provide you with the chance to research the following positions Requirements Lab Technician: Lab Technician Course To enrol in this Lab Technician: Lab Technician Course, students must fulfil the following requirements: Good Command over English language is mandatory to enrol in our Lab Technician: Lab Technician Course. Be energetic and self-motivated to complete our Lab Technician: Lab Technician Course. Basic computer Skill is required to complete our Lab Technician: Lab Technician Course. If you want to enrol in our Lab Technician: Lab Technician Course, you must be at least 15 years old. Career path Lab Technician: Lab Technician Course This training curriculum will be helpful to UK residents looking for new Lab Technician: Lab Technician Course work prospects.
