- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
10885 Professional Development courses in Chester delivered On Demand
Whether you're drawn to supporting others through difficult times or curious about the inner workings of the human mind, this CPD-accredited Counselling Diploma – 3 Courses Bundle offers a solid introduction to modern counselling techniques. With three distinct yet interlinked courses — Counselling Diploma, Life Coaching and Counselling, and Online CBT (Cognitive Behavioural Therapy) — this bundle presents a well-rounded insight into emotional support, mindset management, and behavioural change theory. You’ll explore core counselling approaches, structured life coaching concepts, and CBT methodologies — all delivered in an accessible, online-friendly format. The course is ideal for those seeking a foundational understanding of mental wellbeing strategies, whether for personal development or to enrich existing knowledge. No textbooks to lug around, no overly philosophical waffle — just straight-talking, professionally structured modules built to help you understand the what, why, and how of supporting mental health. If your idea of progress includes helping people untangle their thoughts without having to leave your sofa, this could be the beginning of something meaningful. These comprehensive courses are available in this Counselling Diploma - CPD Accredited 3 Courses Bundle Course 01: Counselling Diploma Course 02: Life Coaching and Counselling Course 03: Online CBT (Cognitive Behavioural Therapy) Course **Special Offer: Free PDF and Hard Copy Certificates** Key Benefits Get instant PDF and Hard Copy certificate Fully online courses Developed by qualified professionals Self-paced learning and laptop, tablet, and smartphone-friendly 24/7 Learning Assistance Course Curriculum: Counselling Diploma Module 01 : Counselling and Counsellors Module 02 : Theories and Models of Counselling (Part 1) Module 03 : Theories and Models of Counselling (Part 2) Module 04 : Legal, Cultural and Ethical Issues in Professional Counselling Module 05 : Forms of Psychotherapy Module 06 : Engaging and Assessing the Patients Module 07 : Helping the Client in Crisis Module 08 : Crisis Intervention Module 09 : Low Intensity CBT Treatment Module 10 : Medication and Therapy in the Treatment of Mental Illness Module 11 : Communication Skills and Empathy in Counselling Course Assessment You will immediately be given access to a specifically crafted MCQ test upon completing each Counselling Diploma - CPD Accredited 3 Courses Bundle bundle course. For each test, the pass mark will be set to 60%. Accredited Certificate After successfully completing this Counselling Diploma - CPD Accredited 3 Courses Bundle course, you will qualify for the CPD Certification Service (CPD UK) certified certificate from Training Express. Disclaimer: The CPD approved course is owned by E-Learning Solutions Ltd and is distributed under license. Why Choose The CPD Certification Service Accredited Course? With 25 years of experience in the Continuous Professional Development sector, CPD Certification Service (CPD UK) is the leading CPD accreditation organisation in the UK, working across all industry sectors. The CPD Certification Service provides recognised independent CPD accreditation compatible with global CPD requirements. Hundreds and thousands of professionals recognise the CPD Certified symbol as the qualitative benchmark that reflects and sets the industry standards. CPD UK evaluates learning activities to the highest standards and courses are certified against the universally accepted structured checklist. CPD 30 CPD hours / points Accredited by The CPD Certification Service Who is this course for? The Counselling Diploma - CPD Accredited 3 Courses Bundle training is ideal for highly motivated individuals or teams who want to enhance their skills and efficiently skilled employees. Requirements There are no formal entry requirements for the course, with enrollment open to anyone! Career path Learn the essential skills and knowledge you need to excel in your professional life with the help & guidance from our Counselling Diploma - CPD Accredited 3 Courses Bundle training.

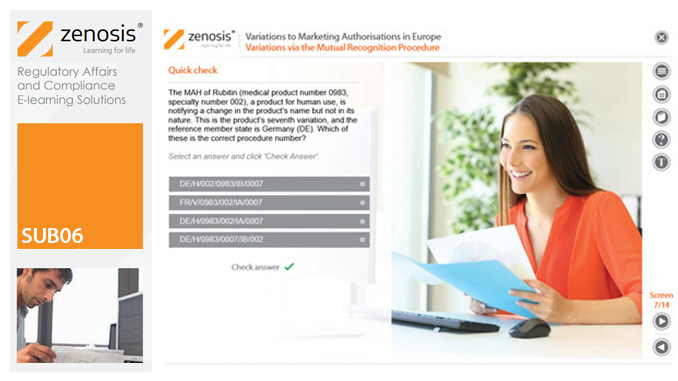
SUB06: Variations to Marketing Authorisations in Europe
By Zenosis
Changes to the terms of marketing authorisations for medicinal products, called variations in Europe, must be notified to or approved by the relevant regulatory authorities. Variations include changes to the composition of products, their manufacturing processes, the way they are used, or the indications for which they are authorised. Common approaches are adopted within the European Economic Area to variations to marketing authorisations approved through the Centralised, Decentralised or Mutual Recognition Procedures. Recent legislation has substantially modified the regulatory requirements and extended them to purely national authorisations by member states. This module, which is fully up to date with the new legislation, covers the classification of variations into their several types and the regulatory requirements, guidance and procedures to be followed for each type.
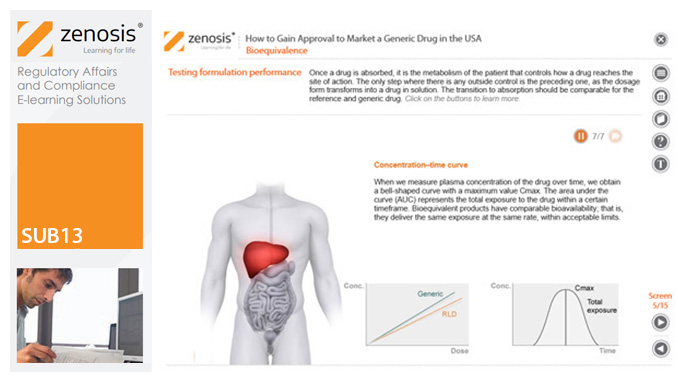
SUB13: How to Gain Approval to Market a Generic Drug in the USA
By Zenosis
This module outlines the legislative and regulatory context for the development of generic drugs and describes the essential role of the Abbreviated New Drug Application (ANDA) in gaining marketing approval. The use of information in the ‘Orange Book’ is explained, as is the role of patent certification in the application. The importance of establishing bioequivalence between a generic and its reference product is emphasised. The module specifies the content and format requirements for an ANDA submission and describes the FDA’s review and approval process. An outline is given of the Generic Drug User Fee Amendments (GDUFA) and the law’s effects on industry players.
SUB14: The Regulatory Pathway to Licensure of Follow-on Biologics (Biosimilars) in the USA
By Zenosis
The regulation of biological medicinal products is governed by different laws from those that apply to small-molecule synthetic drugs. Producing faithful copies of therapeutic proteins is more challenging than producing generic drugs. The US legal framework for the licensure of follow-on biologics, and accompanying regulatory guidance from the Food and Drug Administration (FDA), have been established only in recent years.

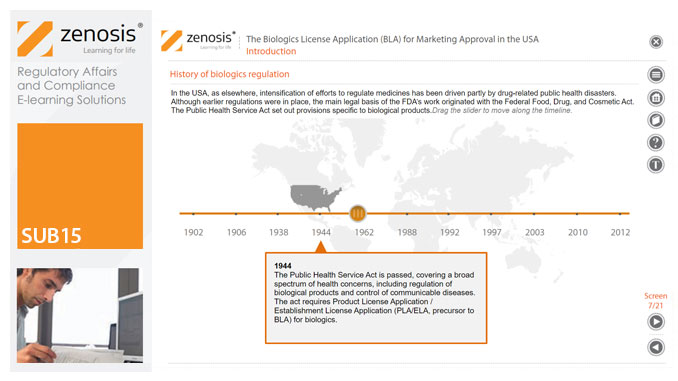
SUB15: The Biologics License Application (BLA) for Marketing Approval in the USA
By Zenosis
This module describes the requirements that must be met to obtain licensure of a biological product. Subjects covered include the regulatory context, the content and format of the BLA submission, the review process, and provisions for expedited development and review.
VAL06: Computer Systems Validation, Part 1: Planning
By Zenosis
In the medicines and healthcare products industries, computerised systems used in automated manufacturing or laboratory processes to which Good Manufacturing Practice requirements apply need to be validated. This module describes the planning of such validation. It follows the work of a pharmaceutical company's team as they validate the dispensary control system for a new production line.

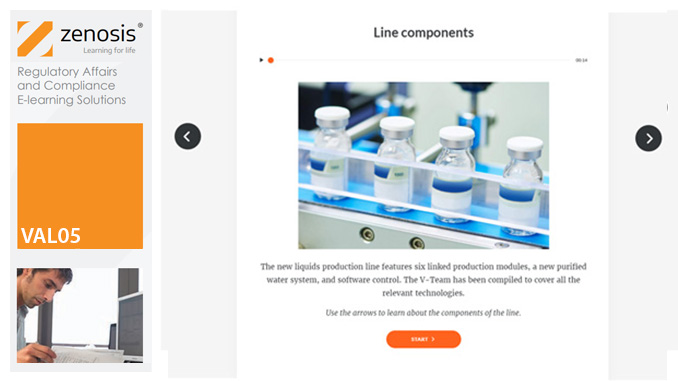
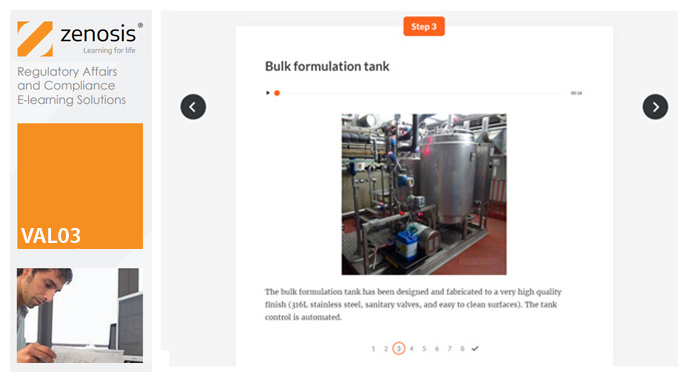
VAL05: Equipment Cleaning Validation
By Zenosis
Manufacturers of medicines and healthcare products must establish, validate and maintain an equipment cleaning programme. This is a regulatory requirement because validated cleaning procedures contribute to the assurance of product purity and safety. This module provides a comprehensive account of equipment cleaning validation requirements and procedures. It follows the work of a pharmaceutical company's validation team as they establish and validate the cleaning program for a new production line.
VAL03: Commissioning and Installation Qualification
By Zenosis
Before equipment can be used routinely in production, it must first be commissioned and, if necessary, undergo Installation Qualification (IQ). This module describes commissioning and IQ requirements and procedures in the medicines and healthcare products industries. It follows the activities of a typical validation team as they carry out a project for a pharmaceutical company.
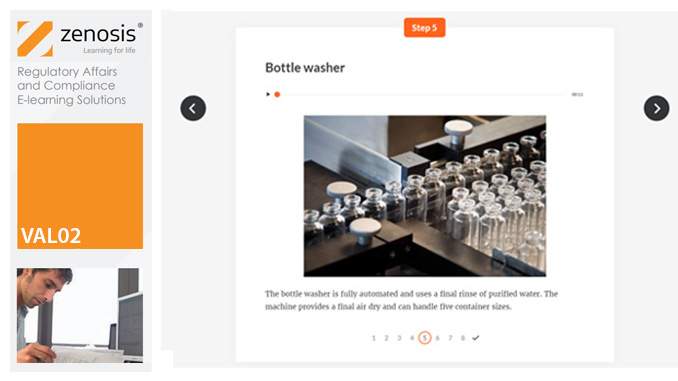
VAL02: Validation Plans and Documentation
By Zenosis
Essential to validation is the provision of documented evidence verifying that manufacturing processes will consistently result in products meeting predetermined quality standards. This module describes the purpose, content and use of validation master plans, project validation plans, and other documentation for validation projects in the medicines and healthcare products industries. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
VAL07: Computer Systems Validation, Part 2: Implementation
By Zenosis
This module describes the design, development and installation phase, the validation phase, and the operation and maintenance phase of the validation of computerised systems in medicines and healthcare products manufacturing environments. It continues to follow the progress of a pharmaceutical company's project to validate a new dispensary control system.