- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
286 Pharmacology courses delivered Online
Medication Training For Carers Mini Bundle
By Compete High
Medication management is a vital part of caring roles, requiring clear knowledge of how medicines work, interact, and affect individuals. This mini bundle covers pharmacy technician basics, biology, chemistry, lab techniques, and essential communication skills. Designed specifically for carers, it offers a rounded introduction to medication concepts and their role in care delivery. From understanding the science behind medications to effectively communicating about medicine schedules, this training supports carers working in diverse environments. Whether managing records or assisting with medication awareness, you’ll gain confidence through structured learning and practical information presented clearly and accessibly. It’s all online, focused, and built around supporting your vital role without any unnecessary jargon. Learning Outcomes: Understand medication types and their effects on the human body. Explore basic biological processes linked to medication use. Recognise chemical principles behind common pharmaceuticals. Learn laboratory techniques related to medication handling. Develop clear communication skills for medication support. Identify safety measures and recording procedures for carers. Who Is This Course For: Carers responsible for medication administration and supervision. Healthcare assistants supporting patient medicine routines. Home care workers assisting with medication reminders. Support staff managing medication records and documentation. New carers seeking structured medication knowledge. Residential care workers handling medicine awareness duties. Health and social care assistants needing basic pharmacology. Anyone supporting clients with medication schedules. Career Path (UK Average Salaries): Healthcare Assistant – £23,500 per year Care Worker – £22,000 per year Medication Support Worker – £25,000 per year Pharmacy Technician Assistant – £27,000 per year Residential Care Officer – £23,000 per year Support Worker – £24,000 per year

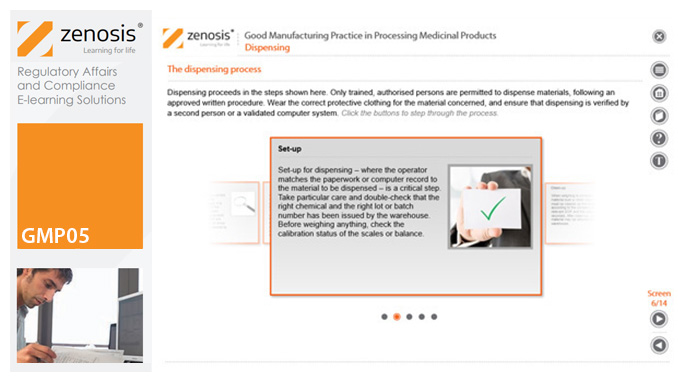
GMP05: Good Manufacturing Practice in Processing Medicinal Products
By Zenosis
Operations in the dispensary and on processing lines are at the heart of medicinal product manufacturing. This module describes how to carry out such operations in compliance with the requirements of Good Manufacturing Practice.
ESS02: Essentials of Monoclonal Antibodies
By Zenosis
This module will introduce you to monoclonal antibodies, explaining how they work, how they are made, and the many uses to which they are put.

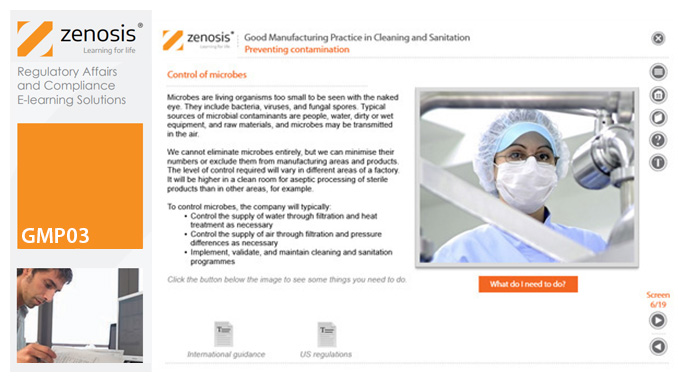
GMP03: Good Manufacturing Practice in Cleaning and Sanitation
By Zenosis
Cleaning and sanitation of premises and equipment are essential to efforts to prevent contamination of product, and they need to be done in compliance with Good Manufacturing Practice (GMP) regulatory requirements. This module shows why it is so important to do a good job, what to consider before and during each job, and how best to go about the work.
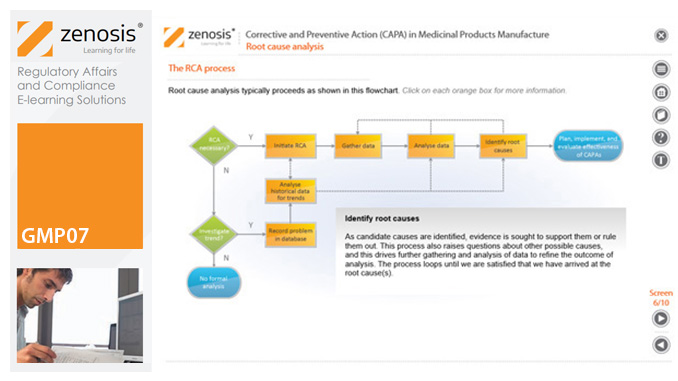
GMP07: Corrective and Preventive Action (CAPA) in Medicinal Products Manufacture
By Zenosis
A company’s Corrective and Preventive Action (CAPA ) system establishes how personnel should deal with manufacturing problems that have occurred or that may occur if not prevented. This module explains the principles of corrective and preventive action and describes typical CAPA procedure. It goes on to introduce root cause analysis and outline the role of progress tracking, escalating, and trending of CAPA procedures.
GMP06: Good Manufacturing Practice in Packaging Medicinal Products
By Zenosis
Packaging for medicinal products is subject to Good Manufacturing Practice rules similar to those for the products themselves. In this module we describe the functions that packaging must fulfil and the quality controls that are applied to packaging materials and operations. We set out the requirements for control of printed materials. We describe preparation, in-process control, and completion of a packaging run. Finally, we explain how to carry out reconciliation of packaging materials.

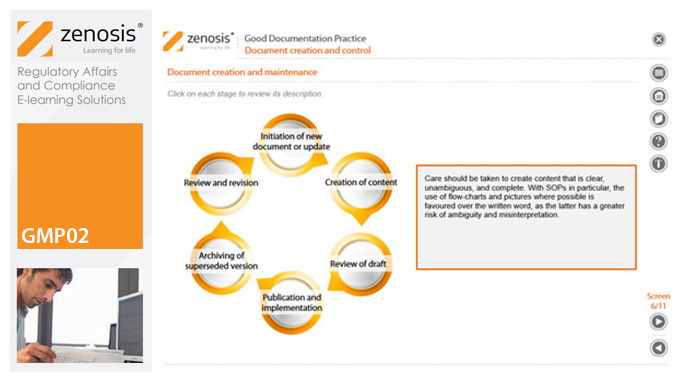
GMP02: Good Documentation Practice
By Zenosis
Good Manufacturing Practice (GMP) for medicinal products relies on documentation. Good Documentation Practice (GDocP) is that part of GMP that applies to the creation, maintenance, use, and retention of documents to provide assurance of the quality of products.
PV06: Urgent Safety Restrictions
By Zenosis
An Urgent Safety Restriction (USR) is a regulatory action taken, in response to a safety signal, to make an interim change to the terms of the marketing authorisation for a medicinal product in Europe. This module describes the principles and procedures for USRs.
GXP01- Good Practices (GxP) in Drug Development and Manufacturing
By Zenosis
This short entry-level module introduces the learner to good practices (GxP) in drug development and manufacturing. It outlines how the industry operates and how it is regulated. It identifies regulatory authorities and other important sources of guidance on Good Manufacturing Practice (GMP), Good Clinical Practice (GCP), and Good Laboratory Practice (GLP).

GXP01: Good Practices (GxP) in Drug Development and Manufacturing
By Zenosis
This short entry-level module introduces the learner to good practices (GxP) in drug development and manufacturing. It outlines how the industry operates and how it is regulated. It identifies regulatory authorities and other important sources of guidance on Good Manufacturing Practice (GMP), Good Clinical Practice (GCP), and Good Laboratory Practice (GLP).
