- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
286 Pharmacology courses delivered Online
CT04a - Clinical trials in drug development
By Zenosis
New drug development requires major investment in capital, human resources and technical expertise. Strict adherence to regulations on testing and manufacturing standards is also required before a new drug can be marketed. One of the greatest challenges in conducting clinical trials is that of efficiency. As trials become more comprehensive, involving large numbers of participants globally, their duration is prolonged and costs increase. The longer trials last, the shorter is the patent life remaining after market approval and the longer patients must wait for the new product. This short course covers the key components of clinical trials and how these requirements interact with the drug development cycle.

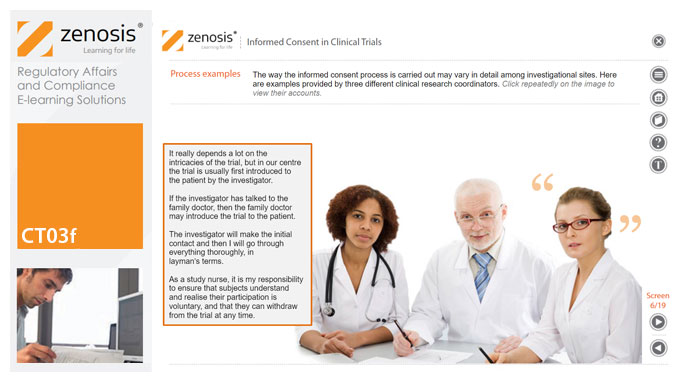
CT03f - Informed consent in clinical trials
By Zenosis
Informed consent in clinical research is an ethical and regulatory requirement. A research subject must enter a study voluntarily, be informed about risks and benefits, and understand the difference between investigation and treatment. Subjects must not be coerced into enrolment, nor must they be enticed by exaggerated claims of benefit. Before they can enrol, all potential subjects must agree, in writing, to participate. In addition to ethical and regulatory imperatives, the potential for litigation by subjects further highlights the importance of rigorous adherence to informed consent principles. In this short course we set out the principles and requirements and provide examples of practical issues confronting healthcare professionals and subjects.
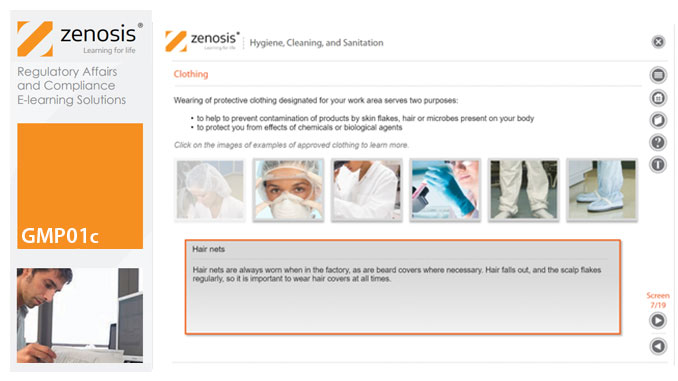
GMP01c - Hygiene, cleaning, and sanitation
By Zenosis
Prevention of contamination is one of the most important goals of GMP. Contamination of product is often difficult to detect, so GMP rules emphasise preventive measures, including: attention to personal health and hygiene, and the wearing of special clothing, by staff; and cleaning and sanitation of premises and equipment. In this short course we set out the basics of GMP requirements in these vital areas.
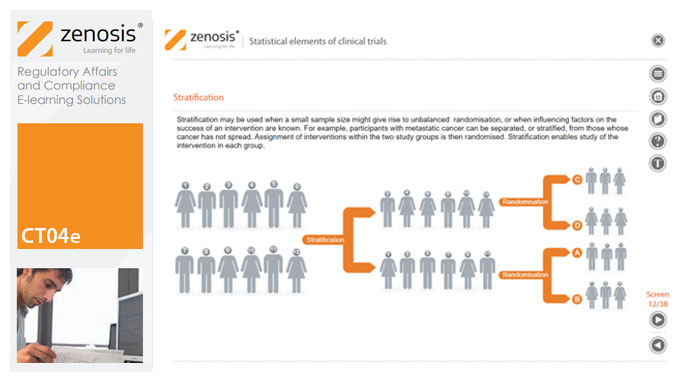
CT04e - Statistical elements of clinical trials
By Zenosis
Analytical statistical elements are essential concepts in the design of clinical trials. This analysis helps us to understand whether a conclusion from a study of a sample of the target population applies generally to that population as a whole. In particular, it helps us to answer the question: Did the treatment effect in the given study occur just by chance? The statistical elements of a well-controlled study minimise the chances of drawing the wrong conclusions, by providing clear thresholds for such errors. The basic statistical elements of a clinical trial include eligibility criteria, randomisation, sample size, power, and blinding, and these are discussed in this short course.
CT04c - Clinical trial preparation
By Zenosis
The demands on quality from clinical trials are increasing. Quantitative aspects of clinical trials, such as the mass of study data to be collected, the multiple investigational sites, and the need to meet predetermined timelines, often supersede qualitative features. Therefore, addressing basic requirements for quality management is essential when preparing a clinical trial. This short course describes the core elements required for the establishment of a clinical trial and provides an overview of the role of the sponsor in supporting and improving trial quality.
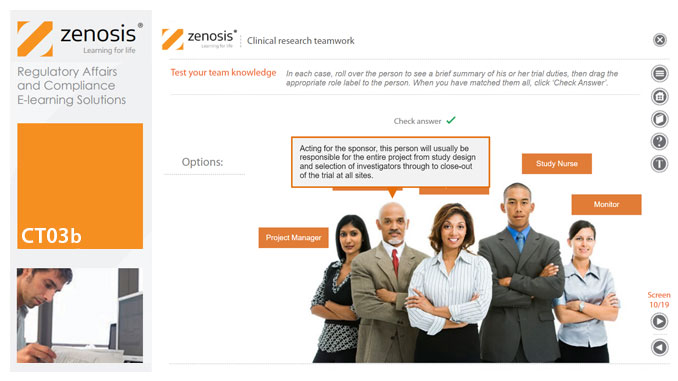
CT03b - Clinical research teamwork
By Zenosis
A clinical trial, particularly a late-phase commercial study, is a major project requiring collaboration between the sponsor and staff or contractor, on the one hand, and the clinical investigator(s) and other healthcare professionals on the other. Good communication among all parties is essential. In this short course we introduce the major roles in a typical clinical research project and outline their duties.
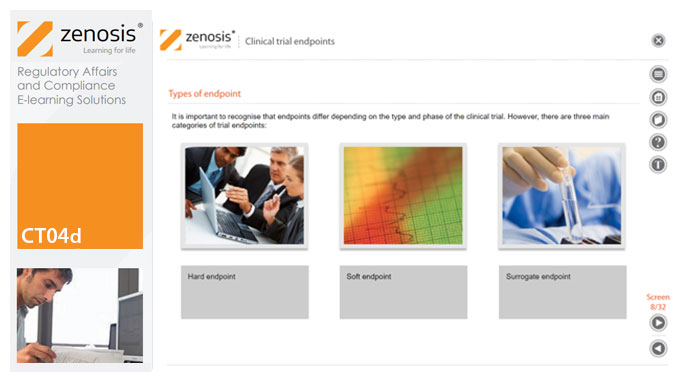
CT04d - Clinical trial endpoints
By Zenosis
In clinical trials, endpoints are measurements to evaluate the results of a new treatment, at an individual patient level. The study data can be extrapolated to patient populations on the basis of clinical similarities to patients participating in the trial. When clinical trial data have been obtained, focus is on the trial endpoints; more specifically, the focus is on whether the trial met or failed the primary endpoint specified before the trial started. The purpose and various types of endpoints are discussed in this short course.
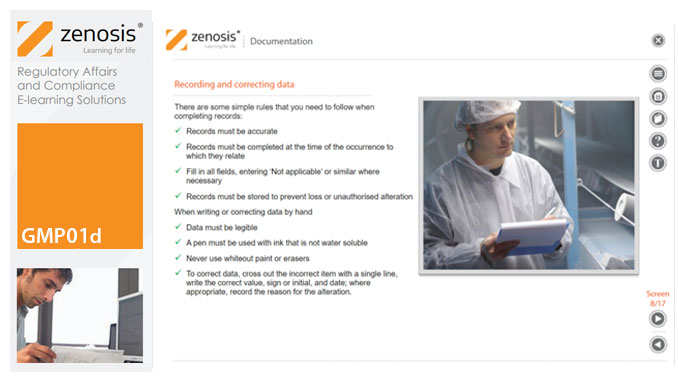
GMP01d - Documentation
By Zenosis
Comprehensive documentation of procedures, formulas, work instructions, and specifications, and thorough recording of batch data, are fundamental requirements of GMP. In this short course we explain why documentation is so important, identify different types of document required, and set out some simple rules for recording and correcting data.
Animal Science Mini Bundle
By Compete High
If you’re serious about working with animals, the Animal Science Mini Bundle is the edge your CV needs. Designed for those targeting jobs in veterinary practice, lab roles, or pet-focused businesses, this bundle covers Animal Care, Biology, Chemistry, Dog Grooming, and Veterinary Assistant skills—all in one job-ready package. Don’t just hope to break into Animal Care or Veterinary Assistant roles—make it happen. Biology and Chemistry roles tied to animal health are growing fast, and Dog Grooming remains a high-demand, low-saturation industry. This is your one chance to gain all five skills before the market catches up. Description The Animal Science Mini Bundle brings together the fields that employers in animal health and pet services look for: Animal Care, Biology, Chemistry, Dog Grooming, and Veterinary Assistant fundamentals. Whether you’re eyeing an entry-level Veterinary Assistant role, a Dog Grooming business, or want to move into Biology or Chemistry-based lab roles in animal science, this bundle gives you five-in-one access to jobs worth applying for. Animal Care skills are a baseline across kennels, rescue centres, and veterinary surgeries. Dog Grooming is booming with private clients and mobile services. Biology and Chemistry experts are sought after in medical research, animal nutrition, and pharmacology. And Veterinary Assistant roles consistently appear on hiring boards. The value? Unmatched. With each of the five core disciplines—Animal Care, Biology, Chemistry, Dog Grooming, Veterinary Assistant—you gain visibility across at least three industries: veterinary, research, and grooming. Whether you're starting your journey or expanding your options, now is the time to jump in. Don’t let a hiring manager pass you over—this bundle helps them notice you. FAQ Q: Is this bundle beginner-friendly? A: Yes. Whether you’re new to Animal Care or exploring Chemistry and Biology as it relates to animals, it suits all levels. Q: What jobs will this help me qualify for? A: Veterinary Assistant, Animal Care Technician, Dog Groomer, Biology Lab Assistant, Chemistry Assistant in pet health sectors. Q: Is Dog Grooming a viable career? A: Absolutely. Dog Grooming is both an in-demand job and a freelance-friendly business opportunity. Q: Does it include lab sciences? A: Yes. Both Biology and Chemistry make this ideal for those seeking scientific roles in animal health. Q: Why include Animal Care and Veterinary Assistant together? A: Because many Veterinary Assistant roles require proven Animal Care competence. This bundle covers both. Q: Can I use this for freelance or mobile work? A: Dog Grooming and Animal Care offer perfect foundations for self-employed services.

Medical Terminology Complete Training - CPD Accredited
By Wise Campus
Medical Terminology Complete Training Would you like to pursue a profession in medical terminology or learn more about it? This medical terminology is intended to help you have a better grasp of medical language. This medical terminology training has covered all the latest topics to keep you better prepared for your medical terminology profession. From introduction to medical terminology to other relevant topics are included in this Medical Terminology Course. Also, you will know about the body systems terminology, medical specialities and procedures in this Medical Terminology course. common medical conditions and diseases are also part of this Medical Terminology course. Through this Medical Terminology course, you can learn to make medical documentation, proper communication and pharmacology. Enrol now for a successful medical terminology career! Main Course: Medical Terminology Free Courses are including with this Medical Terminology Course Along with The Medical Terminology Course, We Offer a free Diploma in Medical Receptionist and Secretary Course Along with The Medical Terminology Course, We Offer a free Clinical Psychology Diploma Course Special Offers of this Medical Terminology Course This Medical Terminology Course includes a FREE PDF Certificate. Lifetime access to this Medical Terminology Course Instant access to this Medical Terminology Course Get FREE Tutor Support to this Medical Terminology Course Medical Terminology Complete Training This medical terminology training has kept you well-prepared for your career by covering all the most recent topics. At the start of our medical terminology course, you will receive an overview of all the important jargon and medical terminology. The Medical Terminology: Medical Terminology Course will teach you different methods of maintaining different body systems. With the help of this medical terminology, you will also be able to manage any common illnesses and medical conditions. Following completion of this medical terminology course, you will be able to keep appropriate medical records and correspondence. Who is this course for? Medical Terminology Complete Training This medical terminology training is open to everyone. Requirements Medical Terminology Complete Training To enrol in this Medical Terminology Course, students must fulfil the following requirements. To join in our Medical Terminology Course, you must have a strong command of the English language. To successfully complete our Medical Terminology Course, you must be vivacious and self driven. To complete our Medical Terminology Course, you must have a basic understanding of computers. A minimum age limit of 15 is required to enrol in this Medical Terminology Course. Career path Medical Terminology Complete Training This medical terminology bundle will help you to get a job in this industry.
