- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
281 Pharmacology courses delivered On Demand
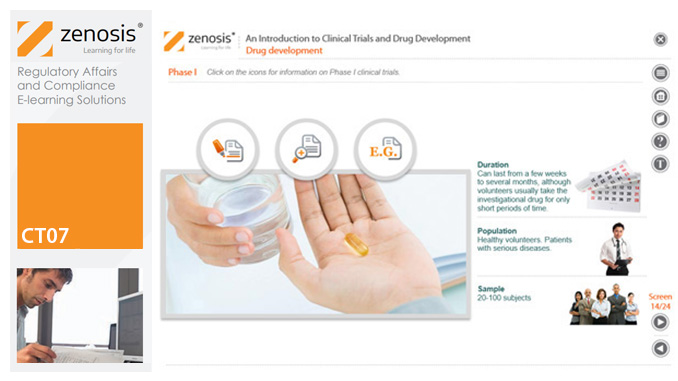
CT07: An Introduction to Clinical Trials and Drug Development
By Zenosis
This module provides an understanding of how clinical trials fit into the drug development process. It outlines the key historical events leading to the development of controlled clinical trials. It specifies the purpose of trials, outlines their features, and identifies codes and regulations that apply to them. Finally, it describes the environment of cost control in which the modern pharmaceutical industry operates.
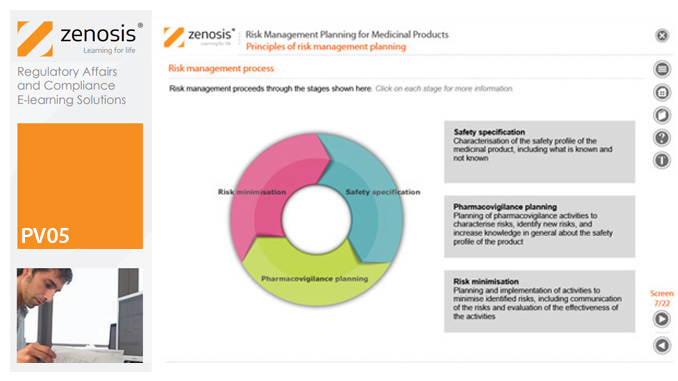
PV05: Risk Management Planning for Medicinal Products
By Zenosis
Proactive risk management is a major component of good pharmacovigilance practice. This module sets out the principles of risk management planning and outlines regulatory requirements for risk management plans in regions that are major markets for medicinal products.
SUB16: The 505(b)(2) Application for Marketing Approval in the USA
By Zenosis
A 505(b)(2) New Drug Application (NDA) is a submission to the Food and Drug Administration (FDA) for approval to market a drug in the USA. It differs from a ‘stand-alone’ NDA in that some of the data on which the applicant relies to demonstrate safety and efficacy have been obtained from publicly available sources rather than from the applicant’s own studies. The applicant typically proposes to market a drug that is based on an approved reference product but modified in its formulation or uses. A 505(b)(2) NDA also differs from an Abbreviated New Drug Application (ANDA) for approval of a generic drug in that the applicant’s product need not be a duplicate of the reference listed drug. The 505(b)(2) pathway may be said to lie part-way between the ‘stand-alone’ NDA and generics pathways, offering a unique combination of advantages to developers. It facilitates the modification of drugs to address unmet medical needs. The 505(b)(2) application pathway accounts for about half of all new drug approvals in the USA.

ICT03: Assuring Data Integrity in Clinical Research
By Zenosis
Pharmaceutical, biotechnology and medical device companies and clinical researchers need to assure regulatory authorities of the reliability of the data that they generate during product development and testing – that is, to demonstrate data integrity. Practices that provide assurance of data integrity in clinical research are required by law and/or established as expectations in regulatory guidance. The data are reviewed in regulatory applications or during regulatory inspections of clinical trial sponsor and investigational sites. Inadequacies of data integrity are frequently reported by inspectors and result in regulatory actions against the organizations or individuals concerned. This course explains the requirements and describes principles and practices that should be followed by trial sponsors, investigators and other clinical research personnel to assure regulators of data integrity.

CT12: How to Conduct Clinical Research Under the EU Clinical Trials Regulation
By Zenosis
This course describes the requirements that must be met by, and options available to, the sponsor during the conduct of an authorised clinical trial. It identifies the various interactions with MSCs that occur via the Clinical Trials Information System (CTIS), and it summarises and links to the extensive guidance available from the European Commission and the European Medicines Agency. Its companion course CT11 sets out the European legal and regulatory context for clinical trials and describes how to apply via the CTIS for authorisation to conduct trials. The two courses therefore provide an ideal foundation for understanding and complying with the new law.

MD01: An Introduction to the Regulation of Medical Devices
By Zenosis
This module provides an introduction to the basics of medical device regulation, especially the requirements that manufacturers must meet in order to market devices in Europe and the USA.

ICT02: Assuring Data Integrity in the Manufacture of Medicinal Products
By Zenosis
Pharmaceutical and biotechnology companies and researchers need to assure regulatory authorities of the reliability of the data that they generate or acquire during product development and manufacturing – that is, to demonstrate data integrity. Data integrity is assessed during regulatory inspections of manufacturing and research sites. Inadequacies of data integrity are frequently reported by inspectors and result in regulatory actions against the companies or individuals concerned. Practices that assure data integrity are required by law and/or expected by regulators in the fields of nonclinical and clinical research, manufacturing and distribution, and pharmacovigilance of medicinal products. This course explains the requirements and describes principles and practices that should be followed to assure regulators and contractual partners of data integrity in the manufacture of medicinal products.
Medication Training For Carers Mini Bundle
By Compete High
Medication management is a vital part of caring roles, requiring clear knowledge of how medicines work, interact, and affect individuals. This mini bundle covers pharmacy technician basics, biology, chemistry, lab techniques, and essential communication skills. Designed specifically for carers, it offers a rounded introduction to medication concepts and their role in care delivery. From understanding the science behind medications to effectively communicating about medicine schedules, this training supports carers working in diverse environments. Whether managing records or assisting with medication awareness, you’ll gain confidence through structured learning and practical information presented clearly and accessibly. It’s all online, focused, and built around supporting your vital role without any unnecessary jargon. Learning Outcomes: Understand medication types and their effects on the human body. Explore basic biological processes linked to medication use. Recognise chemical principles behind common pharmaceuticals. Learn laboratory techniques related to medication handling. Develop clear communication skills for medication support. Identify safety measures and recording procedures for carers. Who Is This Course For: Carers responsible for medication administration and supervision. Healthcare assistants supporting patient medicine routines. Home care workers assisting with medication reminders. Support staff managing medication records and documentation. New carers seeking structured medication knowledge. Residential care workers handling medicine awareness duties. Health and social care assistants needing basic pharmacology. Anyone supporting clients with medication schedules. Career Path (UK Average Salaries): Healthcare Assistant – £23,500 per year Care Worker – £22,000 per year Medication Support Worker – £25,000 per year Pharmacy Technician Assistant – £27,000 per year Residential Care Officer – £23,000 per year Support Worker – £24,000 per year

GMP06: Good Manufacturing Practice in Packaging Medicinal Products
By Zenosis
Packaging for medicinal products is subject to Good Manufacturing Practice rules similar to those for the products themselves. In this module we describe the functions that packaging must fulfil and the quality controls that are applied to packaging materials and operations. We set out the requirements for control of printed materials. We describe preparation, in-process control, and completion of a packaging run. Finally, we explain how to carry out reconciliation of packaging materials.

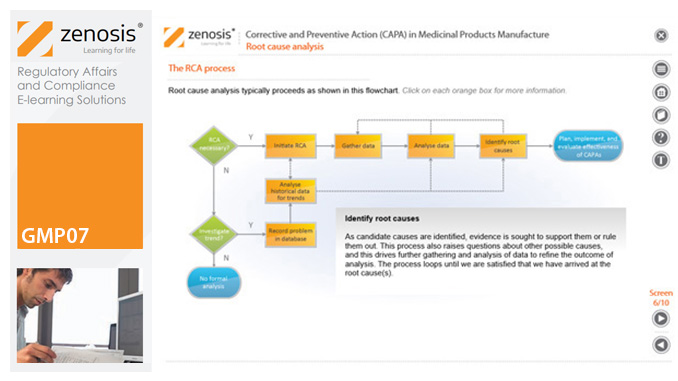
GMP07: Corrective and Preventive Action (CAPA) in Medicinal Products Manufacture
By Zenosis
A company’s Corrective and Preventive Action (CAPA ) system establishes how personnel should deal with manufacturing problems that have occurred or that may occur if not prevented. This module explains the principles of corrective and preventive action and describes typical CAPA procedure. It goes on to introduce root cause analysis and outline the role of progress tracking, escalating, and trending of CAPA procedures.