- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
45170 PE courses
Diploma in Hospitality and Tourism Management - Level 3 (Fast Track)
4.0(2)By London School Of Business And Research
This Diploma in Hospitality and Tourism Management - Level 3 (Fast Track) qualifications (Accredited by Qualifi, UK) have been created to develop and reward the hospitality and tourism managers of today and the future, and to continue to bring recognition and professionalism to the management sectors. Furthermore, we look to develop the team leaders, managers and leaders of the future through the creation and delivery of learning appropriate for industry. The Diploma in Hospitality and Tourism Management - Level 3 (Fast Track) will link to key development in areas of the hospitality and tourism sector. Contemporary issues are identified and evaluated so that the learner has a true vocational understanding on the industry as well as an academic perspective. Key Highlights of this Diploma in Hospitality and Tourism Management - Level 3 (Fast Track) qualification are: Program Duration: Fast Track 6 Months (Regular 9 months duration option available) Program Credits: 120 Designed for working Professionals Format: Online No Written Exam. The Assessment is done via Submission of Assignment Tutor Assist available Dedicated Student Success Manager Timely Doubt Resolution Regular Networking Events with Industry Professionals Become eligible to gain direct entry into relevant Undergraduate degree programme. Alumni Status No Cost EMI Option Requirements Diploma in Hospitality and Tourism Management - Level 3 (Fast Track)This Diploma in Hospitality and Tourism Management - Level 3 (Fast Track) (Accredited by Qualifi) qualifications has been designed to be accessible without artificial barriers that restrict access and progression. Entry to the qualification will be through centre interview and learners will be expected to hold the following: Qualifications at Level 2 AND / OR 1 year work experience and demonstrate ambition with clear career goals OR A Level 3 qualification in another discipline and want to develop their careers in business and entrepreneurship. Career path Learners completing this Course progress to: Level 4 Diploma in Tourism and Hospitality Management, or BA (Hons) in Tourism and Hospitality Management Degree qualification, or Combined Level 4 + Level 5 + Level 6 Diploma in Tourism and Hospitality Management - 360 Credits Undergraduate qualification, or The first year of undergraduate study, or Directly into employment in an associated profession Certificates Certificate of Achievement Hard copy certificate - Included Once you complete the course, you would be receiving a Physical hard copy of your Diploma along with its Transcript which we would Courier to your address via DHL or Royal Mail without any additional charge

TQUK Level 3 Diploma for the Early Years Practitioner (Early Years Educator)
4.5(212)By The Learning College Group
Train to become a Nursery Practitioner, 1-2-1 Tutor support and practical Assessors provided. Easy to use learner platform to guide you through your study step by step. The Level 3 Childcare and Education Diploma (Early Years Educator) is for anyone looking to work in a Nursery as a Nursery Practitioner. This fully recognised Childcare and Education qualification will give you what is required when applying for jobs in Nurseries.

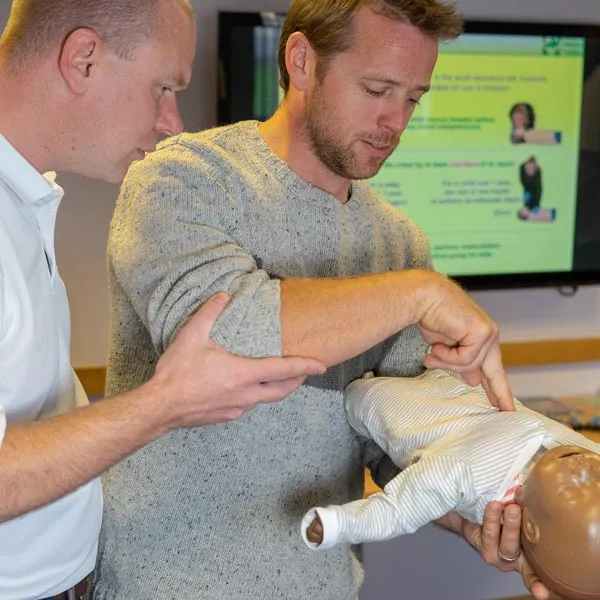
Paediatric First Aid
By Immerse Medical
Our 2 day course will enable students to attain the knowledge and practical competencies needed to deal with a range of first aid situations when looking after children. For more information click on the tabs below, or get in touch, we’d be more than happy to answer any queries. At Immerse Training we pride ourselves on offering First Aid and Pre-Hospital Care Training that meets your specific needs. All our courses meet the requirements of the relevant awarding body. On top of that, we are more than happy to create bespoke elements that tailor each programme to suit your first aid or care responsibilities. Qualification Information This qualification and learning outcomes are based on the recommendations of: The Resuscitation Council (UK) Skills for Health Assessment Principles for First Aid Qualifications Course Content Following this course students will be able to Understand the role and responsibilities of a paediatric first aider. Be able to administer first aid to an infant and a child with head, neck or back injury. Assess an emergency situation safely. Understand how to administer first aid to an infant with conditions affecting the eyes, ears and nose. Be able to provide first aid for an infant and a child who is unresponsive and breathing normally. Understand how to administer emergency first aid to an infant and a child with an acute medical condition or sudden illness. Be able to provide first aid for an infant and a child who is unresponsive and not breathing normally. Understand how to administer first aid to an infant and a child who is experiencing the effects of extreme heat and cold. Be able to provide first aid for an infant and a child who is choking. Understand how to administer first aid to an infant and a child who has sustained an electric shock. Be able to provide first aid to an infant and a child with external bleeding. Understand how to administer first aid to an infant and a child with burns or scalds. Be able to provide first aid to an infant and a child who is suffering from shock. Understand how to administer first aid to an infant and a child who has been poisoned. Understand how to administer first aid to an infant and a child with bites, stings and minor injuries. Understand how to provide first aid to an infant or child with anaphylaxis. Who should attend? This qualification is for people who have a specific responsibility at work, or in voluntary and community activities, to provide first aid to children (including babies) when dealing with: An emergency situation. Chronic or sudden paediatric illness. Paediatric injury. Pre-requisites Students must be at least 16 years old on the first day of training. Assessment and Certifications Assessment of this course is continuous and also includes 2 theory/multiple choice question papers. Successful students will receive an Immerse Training Certificate, which is valid for three years. This certificate will be issued by Qualsafe, the awarding body for Immerse Training. Additional Information Completion of the Level 3 Award in Paediatric First Aid at Work includes 2 credits at Level 2 of the Qualification Credit Framework (QCF). Paediatric First Aid Courses First Aid courses for individuals and workplaces who provide care to or work with children and young people. Our paediatric courses are fully accredited, registered and meet Health and Safety Executive (HSE) and OFSTED guidelines. From 1 day Emergency Paediatric First Aid to day Paediatric First Aid courses. We specialise in on-site courses at your workplace, tailored to the specific risks associated with your business. All courses can be delivered at our training centre in Poole, Dorset or we can deliver on-site across Bournemouth, Poole, Dorset, Hampshire and the South of England.
Thai Yoga Massage works to stimulate, open and balance the flow of energy through the "sen" lines to assist the body in its natural tendency towards self-healing. This is achieved through rhythmic manipulation of sen lines; joint mobilization; passive stretches and applied Yogic postures. Open to all and no specific prerequisites are required. Completion of 5 case studies (4 treatments each) (up to 4 months completion time is allowed after the course) a practical examination lasting two hours. In-class assessment and examinations. Home assignments One day per week, 8 weeks 10.00 - 3 pm

This package is perfect for you if you would like a new look,maybe you are single and want to change your image,or maybe you want a new look for your partner or maybe you want to cheer up a friend that is feeling down but also needs some date coaching and relationship advice and training this is the all in one package. Items included: Relationship coaching session Body confidence training 2-day experience Shopping trip/New wardrobe based on individual’s taste Award-winning celebrity hairdresser Teeth whitening 8 coaching sessions 3 outfits Makeover, drinks, shopping Self-love session , introspection and self acceptance methods Dating advice for singles (Shoe size and dress size must be provided to the M.D.D) Please contact for more details.(03333443853) https://relationshipsmdd.com/product/m-d-d-new-me-makeover/

The Auditing Course
By Research Quality Association
Course Information Designed to develop personal proficiency in audit planning, execution and reporting, this course is meticulously crafted to refine essential audit skill sets. Through immersive scenarios focused on on-site audit conduct (with an alternative Remote Auditing Course available), participants will engage deeply in the audit process. Extending Expertise: Applicable across all audit types, this course builds upon and enriches the foundational concepts taught in RQA's suite of research quality assurance courses. From 'Research Quality Assurance for Good Laboratory Practice' to 'Good Clinical Practice Auditing – Principles and Practice' and 'Good Manufacturing Practice for Investigational Medicinal Products,' this programme extends the scope of learning. Relevance and Value: Relevant to any area of regulated research and development, this course shines particularly in contexts mandating a quality system for audit. Participants with prior audit experience will gain maximum value from this course. Key Benefits: Enrich your skill set to: Navigate audit processes encompassing planning, execution, reporting, and follow-up Embrace a personalised approach fostering positive audit outcomes Analyse evidence and present cohesive audit findings Recognise the pivotal role of audits in driving continual improvement. Interactive Learning: Structured to foster dynamic engagement, this course encourages delegates to: Engage in discussions, idea development, and problem-solving Exchange invaluable information and experiences. Hands-On Experience: A highlight of this course is the series of practical workshops, where delegates work in small syndicate groups, applying the acquired skills from lectures into real-world scenarios. Tutors Tutors will be comprised of (click the photos for biographies): Andrew Waddell Founder Director, Tower Mains Ltd Rosemary Ichaba Senior QA Associate, Tower Mains Ltd Cate Ovington Director, The Knowlogy Group Ltd Jean McWilliam Associate Director, Alexion View pop up Programme Please note timings may be subject to alteration. Day 1 08:45 Registration 09:00 Welcome and Course Objectives 09:10 What is 'Audit'? Delegates explore the range of audits which they have experienced, define the purpose of each audit type and establish which of those audits are performed to meet regulatory requirements. 09:30 Audits and their Purpose The concepts of quality assurance, quality control, quality management and audit are discussed. 10:30 Break 10:45 Audit Planning The requirements for an effective audit programme and individual audit plans. 11:30 Workshop 1 - Getting the Audit Started Planning for the audit. 12:25 Workshop 1 - Feedback 12:45 Lunch 13:30 Workshop 2 - Getting the Audit Started Arranging the opening meeting. 13:50 Workshop 2 - Feedback Audit initiation. Review and discussion of the role of the opening meeting. 14:25 Auditing Techniques (1) - Data and Documentation Techniques for the conduct of data and report audits are investigated. 14:55 Break 15:10 Workshop 3 - Data and Documentation Audit Conducting an audit of a data package and supporting documentation. 17:15 Close of Day Day 2 09:00 Auditing Techniques (2) - The People Questioning techniques which get the required information from the auditee. 09:45 Live Audit Role Play Auditor and auditee behaviours are explored and strategies developed for successful audit interactions. 10:15 Break 10:35 Audit Closing Meeting An exploration of audit closing meetings. 11:00 Workshop 4 - Audit Observations and Preparing for the Closing Meeting Reviewing and categorising your observations and getting ready to present your case. 11:45 Workshop 4 - Feedback 12:30 Audit Reports The content and distribution of an effective audit report are investigated and the importance of effective written communication is discussed. 13:00 Lunch 13:45 Workshop 5 - Audit Reports and Follow-up Mechanisms for promoting effective corrective and preventive action. Critical review of an audit report example. 14:30 Workshop 5 - Feedback 14:55 Corrective and Preventive Action and Follow-up The auditor's role in monitoring responses to audit and the corrective and preventive actions promised is explored. 15:20 Panel Session An opportunity to get answers to outstanding questions. 15:30 Close of Course Extra Information Course material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. CPD Points 14 Points Development Level Develop

Implementing Good Clinical Laboratory Practice
By Research Quality Association
Course Information Join our comprehensive course, meticulously designed to equip individuals implementing Good Clinical Laboratory Practice (GCLP) within laboratories handling samples from clinical trials. Delve into the current regulatory landscape governing laboratory work supporting clinical trials, referencing key guidelines such as the ICH Guideline for Good Clinical Practice, the Clinical EU Trials Directive, relevant regulations, and leveraging insights from the RQA guidance document on GCLP. Is this course for you? This course is tailored for laboratory managers, analysts, investigators, trial coordinators, monitors, and auditors operating in diverse settings such as pharmaceutical company laboratories, central laboratories, contract research organisations, hospital laboratories, clinics, and investigator sites. This course will give you: Guidance on effectively interpreting and applying GCLP within the broader framework of Good Clinical Practice (GCP) Insight into the seamless integration of GCLP within clinical programmes (GCP) Practical strategies for implementing GCLP in the nuanced environment of clinical research laboratories The chance to update your knowledge with the latest interpretations and guidance on clinical laboratories by the UK Medicines and Healthcare Products Regulatory Agency (MHRA) Access to a seasoned panel of speakers with extensive expertise A unique opportunity to deepen your understanding of GCLP's application across diverse scenarios. Engage in: Lively discussions to foster ideas Problem-solving sessions targeting specific challenges Detailed exploration of specific aspects within the realms of GCP and GCLP. Tutors Tutors will be comprised of (click the photos for biographies): Vanessa Grant -, - Louise Handy Director, Handy Consulting Ltd Tim Stiles Consultant, Qualogy Ltd Programme Please note timings may be subject to alteration. Day 1 08:50 Registration 09:00 Welcome and Introduction 09:20 Good Clinical Practice and the Requirements of Good Clinical Laboratory Practice A review of Good Clinical Practice and its requirements for the laboratory analysis of samples from a trial. The thought processes behind the development of Good Clinical Laboratory Practice, its objective, scope, interpretation and application are explained. 10:00 Safety and Ethical Consideration Informed consent, confidentiality, expedited reporting, blinding and unblinding and serious breaches of the GCP are discussed. 10:40 Break 10:55 Organisation and Personnel Responsibilities within GCP and the Laboratory The responsibilities of key functions that should exist within a clinical laboratory including personnel records of training and competence are discussed. 11:30 Staff Training and Training Records Personnel records of training and competency assessments are discussed. 11:45 Laboratory Facilities, Equipment and Materials Suitable facility design, organisation and operation will be discussed. The calibration, validation and maintenance of equipment used in the conduct of sample analysis are examined, as are the suitability of materials and the identification and labelling of reagents and solutions. 12:30 Lunch 13:15 Workshop 1 - Facilities, Equipment and Responsibilities Some practical problems with regard to the facilities, equipment and responsibilities are explored. 13:45 Workshop 1 - Feedback 14:15 Computer Systems Validation Systems, including computerised systems, used in the analysis, collection and reporting of results should be appropriately tested, operated and controlled. What this means in practice is discussed. 14:45 Trial Protocols, Analytical Plans During this session we examine the purpose, content, control and change of these important documents. 15:30 Break 15:45 Workshop 2 - SOPs, Clinical Protocols, Analytical Plans and Validation The practicalities of managing and documenting the planning phase of analytical work on a trial are explored along with computerised system validation. 16:30 Workshop 2 - Feedback 17:00 Close of Day Day 2 09:00 Conduct of the Work and Quality Control Many of the issues that surround the conduct of sample collection, shipment, storage, analysis and management of Analytical Methods are discussed. This includes the quality control of the assay that may be employed and Quality Control checks. 10:00 Deviation Management The expectations around deviations and CAPA are discussed. 10:15 Workshop 3 - Conduct of the Work and Quality Control Practical work conduct and quality control issues are explored. 10:45 Break 11:00 Workshop 3 - Feedback 11:30 Source Data, Data Integrity, Records and Reports The creation and subsequent management of source data and records, data integrity, are discussed, together with the process of reporting analytical results. 12:10 Workshop 4 - Data, Records and Reports Practical problems with data, records and reports are investigated. 12:45 Lunch 13:30 Workshop 4 - Feedback 14:00 Quality Audit The requirements for and purpose of quality audits are discussed. The difference between quality audit and quality control are explained along with the role of the quality audit staff and their interaction with the analytical project managers, laboratory management and study staff. 14:40 Risk Management How should we assess risk and how can we use the process to assist in evaluation of audit findings. 15:15 Break 15:30 Regulatory Inspection The conduct of regulatory inspections and current expectations of the inspectors. Preparation for inspections and conduct during them will be discussed. 16:00 Panel Session This panel session will address any outstanding issues raised by the delegates. 16:15 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Develop

Microsoft Azure Expert Certification Bundle (with 3 Exams)
By Hudson
The Microsoft Certified Expert is a new breed of Microsoft certification. It is referred to as a ‘role-based certification’. According to Microsoft, role-based certifications show that individuals that possess them are keeping pace with today’s technical roles and requirements. They allow a learner to skill up and prove their expertise to employers and peers, plus get the recognition and opportunities they’ve earned.

Microsoft Azure Bundle with 3 Exams (3 Certifications)
By Hudson
The Microsoft Certified Fundamentals, Associate, and Expert is a new breed of Microsoft certification. It is referred to as a ‘role-based certification’. According to Microsoft, role-based certifications show that individuals that possess them are keeping pace with today’s technical roles and requirements. They allow a learner to skill up and prove their expertise to employers and peers, plus get the recognition and opportunities they’ve earned.

M.D.D I WANT MY EX GIRLFRIEND BACK PACKAGE (MEN DATING SERVICES$
4.9(27)By Miss Date Doctor Dating Coach London, Couples Therapy
Coach contacts your ex Mediation Discuss issues Relationship training Eliminate negative patterns from the relationship Date arranged at a 5-star venue Gift package sent to the girlfriend 5 sessions 45 mins per session https://relationshipsmdd.com/product/i-want-my-ex-girlfriend-back-package/

Search By Location
- PE Courses in London
- PE Courses in Birmingham
- PE Courses in Glasgow
- PE Courses in Liverpool
- PE Courses in Bristol
- PE Courses in Manchester
- PE Courses in Sheffield
- PE Courses in Leeds
- PE Courses in Edinburgh
- PE Courses in Leicester
- PE Courses in Coventry
- PE Courses in Bradford
- PE Courses in Cardiff
- PE Courses in Belfast
- PE Courses in Nottingham