- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
BEHAVIORAL INTERVIEWING: BUILDING A CONSISTENT FRAMEWORK AND PROCESS
5.0(4)By Improving Communications Uk
LEARN ABOUT BEHAVIORAL VS. TRADITIONAL INTERVIEWING, AND HOW TO INCORPORATE AND DEVELOP INTERVIEWING SKILLS TO ENSURE THAT YOU FIND THE RIGHT CANDIDATE FOR THE JOB. Behavioral Interviewing means asking candidates questions that will help you to discover how the interviewee acted in specific employment-related situations. Because past performance is a good indicator of how someone will act in the future, this style of interviewing is extremely useful, and the method of choice for recruiting teams. In this session, you will learn about behavioral vs. traditional interviewing, and how you can incorporate and develop your interviewing skills to ensure that you have the right candidate for the job. OBJECTIVES Participants will be able to: Build a consistent framework and process to ensure an unbiased candidate experience; Choose job specifications and determine how success will be measured (skills); Identify characteristics and qualities that will support the required skills; Prepare questions to elicit descriptions of behaviors, attitudes, and skills necessary for the job; Review legal and appropriate interviewing etiquette/guidelines, including social media research; Screen candidates, using resumes and phone interviews; and Conduct successful role-play Behavioral Interviews in class. CLASSES WILL INCLUDE: Workbooks for future reference and study. Workshop / role play with actual interview scenarios to assist in internalizing data. Time for individual questions and concerns to aid in personalizing tactics. Online Format—Behavioral Interviewing is a 4-hour interactive online class for up to six people. Register for this class and you will be sent ONLINE login instructions prior to the class date. Rich has an engaging presentation style. The New Mexico chapter of the International Society for Performance Improvement (NMISPI) gave high marks to his interactive and lively Improving Customer Service workshop. There were opportunities to share ideas and analyze different techniques, and 87% of attendees said that they would recommend this workshop to others. Ildiko OraveczNew Mexico International Society for Performance Improvement

Overview Did you know that the global HR industry is currently worth around £17,48 billion? Get a chance to shine in this highly prospective and competitive field through our HR Management bundle. Our precisely crafted courses will help you establish yourself in this field! HR is more than just a division of a company. It is essential to any business and worker's success and is the lifeblood of an organisation. The Human Resources principles covered in this CPD-accredited course will adequately equip you to begin a career in the related industry. For organisations of all sizes, managing daily HR tasks is a difficult undertaking that needs the assistance of an HR consultant. Moreover, this course will give you the essential knowledge you need to create individualised training plans and effectively handle human resources-related demands. Following your successful completion, you will have highly developed abilities that will enable you to design and manage the recruiting and selection process, organise and carry out training, implement performance improvement training, and more. Along with this HR Management Complete Bundle, you will get an original hardcopy certificate, a transcript and a student ID card which will allow you to get discounts on things like music, food, travel and clothes etc. What other courses are included with this Bundle? Course 1: Level 3 Diploma Course 2: HR And Recruitment Consultant Training Course 3: Employment Law Course 4: Office Administration Certificate Course 5: Contract Manager Diploma Course 6: Payroll Management Course Course 7: GDPR Course 8: Workplace Confidentiality Course 9: Leadership & Management Diploma Course 10: Performance Management Course 11: Effective Communication Skills Learning Outcomes The students undertaking this course will get the opportunity to - Get Introduced to Human Resources (HR) Understand the employee recruitment and selection procedure Learn the skills of an effective administrator Understand the employee training and development process Learn about performance appraisal management and employee relations Benefits you'll get choosing Apex Learning: One payment, but lifetime access to 11 CPD courses Certificates, student ID for the title course included in a one-time fee Full tutor support available from Monday to Friday Free up your time - don't waste time and money travelling for classes Accessible, informative modules taught by expert instructors Learn at your ease - anytime, from anywhere Study the course from your computer, tablet or mobile device CPD accredited course - improve the chance of gaining professional skills Gain valuable experience without leaving your home How will I get my Certificate? After successfully completing the course you will be able to order your CPD Accredited Certificates (PDF + Hard Copy) as proof of your achievement. PDF Certificate: Free (Previously it was £6*11 = £66) Hard Copy Certificate: Free (For The Title Course: Previously it was £10) Description Begin your HR journey with Apex Learning right away! The management of human resources is essential to the success of any company and employee. In addition to ensuring regulatory compliance, HR is in charge of keeping staff members interested and inspired to perform well. This course will provide you with the overview required to achieve your objective, whether you are entirely new to human resources, are interested in learning more about the field, or need to create a more effective HR department. Additionally, students will study about the evolving function of HR, including a historical review, important procedures and duties, and contemporary trends influencing the development of HR practices. With the help of this HR Management Bundle, you will get the core knowledge necessary to create customised training plans and effectively handle human resources-related demands.When you need them, knowledgeable teachers and mentors will be available to you and will answer any of your questions via email and chat rooms. Course Curriculum of Bundle Module 01: Introduction to Human Resource In this module we will cover the following topics: Human resource management Models of HRM The Role of the HR Department Aims of HRM HRM and personnel management Impacts HR have on Organisational Performance Module 02: Employee Recruitment and Selection Procedure In this module we will cover the following topics: Attracting candidates Advertising E-recruitment Graphology Final stages Module 03: Employee Training and Development Process In this module we will cover the following topics: The justification for training Transferring training Systematic training Types of training Effective training practices Training techniques E-learning Module 04: Performance Appraisal Management In this module we will cover the following topics: The benefit of performance management Characteristics of performance management Performance management cycle Managing performance throughout the year Reviewing performance Rating performance Dealing with under-performers Module 05: Employee Relations In this module we will cover the following topics: Strategic Employee Relations Practice of Industrial Relations Employee Voice Employee Communications Module 06: Motivation and Counselling In this module we will cover the following topics: The process of motivation Types of motivation Motivation theory Motivation strategies Module 07: Ensuring Health and safety at the Workplace In this module we will cover the following topics: Managing Health and Safety At Work The importance of health and safety in the workplace Benefits of workplace health and safety Health and safety policies Conducting risk assessments Accident prevention Measuring health and safety performance Health and safety training Module 08: Employee Termination In this module we will cover the following topics: Redundancy Dismissal Relieving Letter Retirement Module 09: Employer Record and Statistics In this module we will cover the following topics: Introduction The necessity for Personnel Records Information in Personnel Records Formats of Personnel Records Module 10: Essential UK Employment Law In this module we will cover the following topics: Introduction Wages Dismissal Equality Working Parents' Rights Data Protection (GDPR) Course 2: HR And Recruitment Consultant Training Introduction to Recruitment: Importance and Implications An Overview of the Recruitment Industry The UK Recruitment Legislation Sales and Selling in the Recruitment Industry The Recruitment Process Key Performance Indicators for the Recruitment Industry Candidate Attraction for Recruitment Candidate Management Candidate Interview Processes And many more... Course 3: Employment Law Basic of Employment Law Legal Recruitment Process Employment Contracts Employee Handbook Disciplinary Procedure National Minimum Wage & National Living Wage Parental Right, Sick Pay & Pension Scheme Discrimination in the Workplace Health & Safety at Work Dismissal, Grievances and Employment Tribunals Workplace Monitoring & Data Protection Course 4: Office Administration Certificate Introduction to Office Administration Skills of an Effective Administrator Business Writing Skills Communication Skills Managing Meetings and Giving Feedback Organisational Skills Telephone Etiquette Negotiation Techniques Conflict Management Stress Management Time Management Course 5: Contract Manager Diploma Introduction to Contract Management Classification of Contracts Contract Lifecycle - Understanding The Stages Principles of English Contract Law Contract Management Plan & Efficiency Contract Manager's Roles, Responsibilities & Career Opportunities And many more... Course 6: Payroll Management Course Payroll System in the UK Payroll Basics Company Settings Legislation Settings Pension Scheme Basics Pay Elements The Processing Date Adding Existing Employees Adding New Employees Payroll Processing Basics Entering Payments Pre-Update Reports Updating Records e-Submissions Basics Process Payroll (November) Employee Records and Reports Editing Employee Records Process Payroll (December) Resetting Payments Quick SSP An Employee Leaves Final Payroll Run Reports and Historical Data Year-End Procedures Course 7: GDPR GDPR Basics GDPR Explained Lawful Basis for Preparation Rights and Breaches Responsibilities and Obligations Course 11: Workplace Confidentiality Module 01: Introduction to workplace confidentiality Module 02: Business Etiquettes and Types of Confidentiality Module 03: The Importance of Confidentiality Module 04: Confidentiality with Co-workers Module 05: Preventing Confidentiality Breach Module 06: How Employers Can Protect Confidential Information Course 9: Preventing Workplace Violence and Harassment Section 1: Workplace Management: Violence What Is Workplace Harassment? Identifying the Bully How to Handle Workplace Violence Risk Assessment Being the Victim Checklist for Employers Interview Process Investigation Process Developing a Workplace Harassment Policy Section 2: Workplace Management: Harassment The Background Developing an Anti-Harassment Policy Policies in the Workplace Proper Procedures in the Workplace False Allegations Other Options Sexual Harassment Mediation Conflict Resolution The Aftermath Course 10: Performance Management Performance Management Introduction Performance Key Performance Indicators Reporting Rewarding Conclusion Course 11: Effective Communication Skills Business Communication Verbal and Non-verbal Communication Written Communication Electronic Communication Communicating with Graphics Effectively Working for Your Boss CPD 110 CPD hours / points Accredited by CPD Quality Standards Who is this course for? This course bundle is designed for people who are - Looking to pursue a career in Human Resourse (HR). Willing to upgrade their skills. Trying to build an efficient workforce in HR. Working on boosting office productivity. Willing to ensure an employee friendly workplace. Requirements Our HR Management(HR) course is fully compatible with PCs, Macs, laptops, tablets and Smartphone devices. Career path HR has a huge job field. This course bundle will help the learners to pursue jobs that have huge demand in the job market, such as- Human resources officer Equalities officer Human Resources Assistant. HR Specialist Administrative Officer

Python for Machine Learning - The Complete Beginner's Course
By Packt
The purpose of this course is to teach you how to use Python for machine learning to create real-world algorithms. You will gain an in-depth understanding of the fundamentals of deep learning. This course will help you explore different frameworks in Python to solve real-world problems using the core concepts of deep learning and artificial intelligence.

CONSULTATIVE SELLING Training Programme Framework
By Dickson Training Ltd
Key Objectives for the Training provision Effective listening to a client's needs and requirements during various stages of the sales process. Engage in meaningful communication with clients, learn to identify challenges and opportunities that relate to the prospects. Overcome the identified challenge. Build long term rapport and establish trust with the prospect throughout the sales process, Ensure continued customer satisfaction that can turn into repeat business. Be able to build rapport with customers or clients. Know the right questions to ask to fully understand the customer or client’s needs without putting on any pressure. Be able to check you have the right information from the customer or client. Be able to match products and services to the customer or client using the information you have gathered. Be able to influence effectively. Know how to stand out from your competitors. Stage 1 – Research the Company’s ‘Value Propositions’ and USP Analysis Conducting 30 min confidential interviews with a cross-section of the eligible Attendees & the Stakeholders, in order to gain an objective understanding of the various scenarios and clients that the Participants work with. the Company’s ‘Value Propositions’ Unique Selling Points The Customers’ journey(s) The Competition’s offers for analysis and comparisons. From these findings, a carefully calibrated bespoke Programme will be designed and delivered. Stage 2 - Design the Bespoke 2 x day Course nd Stage 3 - Delivery of the Course Programme This Programme can be delivered in 2 x consecutive days, or split between 2 – 3 weeks, to make it more ‘work-friendly’ – if required. It is very practical and commercially focussed in approach, with lots of interactive exercises designed to draw out the learning applications via the debriefings. The ‘Real Play’ scenarios on day 2 will be based on specific case studies drawn from the Research findings so that they are authentic to optimise the learning. Individual Action Plans will be captured at the end of each day to be debriefed by the Participants’ respective Line Managers. Template Programme Day One – Foundation – Strategic Approach Section One – The Principles of Consultative Selling Defining ‘Consultative Selling’ The Company’s ‘Unique Selling Points’ & Value Proposition The Consultative Selling model – the five stages Avoiding appearing ‘pushy’, ‘pressurising’ or ‘talking through the sale’ Section Two –Strategy & Preparation ‘Buy-Class’ Matrix Strategic plan Template – matching services/products to identified needs & opportunities. Preparing a range of objectives Constants and variables – USP’s Researching key Client/Decision Makers’ priorities, profile & background (LinkedIn etc.) Preparing for potential & likely Objections Section Three – Email etiquette & ‘influencing’ email correspondence. Email etiquette A.B.S.U.R.D model Clarity and tone – discovery of clients’ needs & motivational factors. Techniques to influence and ‘nudge’. Ensuring the data and content have a ‘gentle’ motivational & appealing message. Top Tips Day Two – Practical Application Section Four – Rapport Building & Effective Communication skills ‘Behaviour Labelling’ techniques – setting a positive tone. Asking Open Questions – gaining a full understanding of the clients’ priorities & expectations. EQ - Inviting opinions; perspectives; experiences – winning confidence. Active Listening – focus and commitment to understand. ‘Reading the room’ – adapting to responses and reactions. Maintaining focus on relevant topics that the Client cares about; carefully consider the opportunities to influence. But NO PRESSURE! Building a ‘bond of trust’ with commitments and authentic, shared values. Section Five – Explaining the Benefits – aligned to the Customers’ Priorities Understanding ‘Why do People Buy?’ Research data analysis. Open questions that lead to understanding the key issues and Clients’ priorities. Avoiding the danger of ‘pressurising’ the Client. Responding to objections effectively, with confidence and sensitivity. Explaining the benefits that are aligned to the Clients’ stated requirements. Making the Data ‘sing’ – memorable takeaways for the Client. Augmented benefits – Brand confidence; Case studies; Warranty; Service; Range; Flexibility etc. in alignment with their stated preferences/requirements Differentials compared to Competitors Section Six – Winning Commitment Inviting commitment – with confidence Consultative Selling Closing techniques (e.g. Alternative Close/Assumptive Close et al) Avoiding ‘talking through the sale’ – knowing when to ‘Shut UP!’ Confirming agreement – ensuring Clients’ motivation for repeat business Section Seven – Practical Application ‘Real Play’ From the Research findings, carefully devised scenarios can be generated to provide the most valuable learning opportunities to underpin all the skills & techniques covered in the Programme. Potential Real Play scenarios: A well-established customer has indicated that they are soon to be opening up additional new offices – this presents an opportunity to arrange for the Company to support them with their upcoming needs. After some very positive feedback and with the upcoming contract renewal imminent – how can the Company give the Customer more high-quality support in other areas of their business? A Competitor has been to see the client and they have prompted some concerns about ‘value for money’ & ‘quality of service’. How Real Play works… The group is split the group into 2 sub-groups, one with our Professional Actor (option available); the other with the Trainer or a willing Participant. Each group has a brief and has to instruct their Trainer/Actor/Participant on how to approach the scenario supplied. The Actor and Trainer (or willing Participant) perform the role play(s) as instructed by their respective teams; however, during the action they can be paused for further recommendations or direction. The outcome is the responsibility of the team(s) – not the performers. Debrief the full Programme Individual Action Plans - to be followed through. ‘Best Practices’ for application into the business Options for Exercises within the Programme Sample Exercise – Red & White There is a specific time managed agenda and itinerary, which puts the group under pressure. The key challenge is for the sub-groups to maximise the commercial value from the task, however there is always a great danger that the individuals attempt to gain financial progress at the expense of the other group! Debriefing points: Persuasive communication and influence across barriers Gaining buy-in when others are sceptical Strategic planning accounting for others’ behaviours Sample Exercise – Communication Challenge Each Participant has different pieces of information, but are not allowed to share it visually. They are only allowed to communicate to work out the solution hidden among the large amount of data. Debrief: Structured approach Maintaining focus through distractions/interference Active Listening Controlled communication Sample Exercise – Persuasive emails Sample emails are shared to be critiqued and improved upon to be debriefed: Tone & impact Making the Data ‘sing’ Influential & motivational language Customer centric message. Sample Exercise –Juggling Each Participant has to pass the ‘Customers’ (Juggling balls) carefully through the system with all the other Participants to reach a profitable conclusion Debrief: EQ to read the room and effectively communicate, when the pressure is on Customer focus and adapting to challenges Devising a plan that wins buy-in Overcoming competing ideas to get to ‘best practice’ Consistent commitment, communication and motivational drivers that influenced performance. Sample Exercise –Critical Path The group are provided with 30 x discs and some ‘post-it’ notes. (no pens or pencils are allowed) Their brief is to create a grid shape with the 30 x discs, which will act as ‘stepping stones’ for the ‘Critical Path’ But they have to follow the correct order through the grid from the start to finish, which they will have to work out through ‘trial & error’ All of the team must pass through the ‘Critical Path’ worked out on the grid, observing the constraints, within the time limit. No talking is permitted once they start using the materials Debrief: - Clear communication focussing on the ‘client’s journey’ Planning for challenges Identifying risks and mitigating them Role allocations & support Quality control and disciplines Sample Exercise –Back to Back Each Participant is positioned back-to-back with a partner. They have to work out precisely what the ‘statement’ given to the other person is without looking around. Each person has a different brief. Debrief: - Asking Open Questions Active Listening Accuracy in identifying the objective.

Digital CCTV and Remote Access Course
By Hi-Tech Training
The Digital CCTV & Remote Access course is designed to give participants a practical knowledge of integrating Analogue and Digital technologies in addition to access and control from remote locations such as laptops, tablets and mobile phones.

In this competitive job market, you need to have some specific skills and knowledge to start your career and establish your position. This Management Skills: New Manager course will help you understand the current demands, trends and skills in the sector. The course will provide you with the essential skills you need to boost your career growth in no time. The Management Skills: New Manager course will give you clear insight and understanding about your roles and responsibilities, job perspective and future opportunities in this field. You will be familiarised with various actionable techniques, career mindset, regulations and how to work efficiently. This course is designed to provide an introduction to Management Skills: New Manager and offers an excellent way to gain the vital skills and confidence to work toward a successful career. It also provides access to proven educational knowledge about the subject and will support those wanting to attain personal goals in this area. Learning Objectives Learn the fundamental skills you require to be an expert Explore different techniques used by professionals Find out the relevant job skills & knowledge to excel in this profession Get a clear understanding of the job market and current demand Update your skills and fill any knowledge gap to compete in the relevant industry CPD accreditation for proof of acquired skills and knowledge Who is this Course for? Whether you are a beginner or an existing practitioner, our CPD accredited Management Skills: New Manager course is perfect for you to gain extensive knowledge about different aspects of the relevant industry to hone your skill further. It is also great for working professionals who have acquired practical experience but require theoretical knowledge with a credential to support their skill, as we offer CPD accredited certification to boost up your resume and promotion prospects. Entry Requirement Anyone interested in learning more about this subject should take this Management Skills: New Manager course. This course will help you grasp the basic concepts as well as develop a thorough understanding of the subject. The course is open to students from any academic background, as there is no prerequisites to enrol on this course. The course materials are accessible from an internet enabled device at anytime of the day. CPD Certificate from Course Gate At the successful completion of the course, you can obtain your CPD certificate from us. You can order the PDF certificate for £4.99 and the hard copy for £9.99. Also, you can order both PDF and hardcopy certificates for £12.99. Career path The Management Skills: New Manager will help you to enhance your knowledge and skill in this sector. After accomplishing this course, you will enrich and improve yourself and brighten up your career in the relevant job market. Course Curriculum Section 01: Introduction Course Introduction 00:03:00 Course Aims 00:03:00 A Summary of the 21 Strategies 00:07:00 Section 02: The 21 Strategies for Success as a New Manager Strategy 1. Get the Lie of the Land 00:10:00 Strategy 2. Create a Vision 00:06:00 Strategy 3. Know Your Area of Responsibility 00:07:00 Strategy 4. Determine if Delivery Is Happening 00:11:00 Strategy 5. Create Allies & Keep Them 00:08:00 Strategy 6. Get a Mentor 00:07:00 Strategy 7. Know What Your Customers Want & Give It to Them 00:06:00 Strategy 8. Secure Early Wins & Pick Low-Hanging Fruit 00:07:00 Strategy 9. Define the A-List Priorities 00:06:00 Strategy 10. Live For the Team 00:11:00 Strategy 11. Don't Make Silly Mistakes 00:06:00 Strategy 12. Get Onside With the Boss 00:09:00 Strategy 13. Be an Agent for Change 00:09:00 Strategy 14. Focus, Focus, Focus! 00:06:00 Strategy 15. Understand the Culture 00:06:00 Strategy 16. Don't Let Method Undermine Objective 00:05:00 Strategy 17. Put Goals in Place 00:07:00 Strategy 18. Know the Plan, Hit the Plan 00:13:00 Strategy 19. Instil Behavioural Change 00:10:00 Strategy 20. Deliver. Plain and Simple 00:11:00 Strategy 21. Celebrate the Wins 00:12:00 Bonus Module: Strategy 22. Maintain Team Cohesion While Apart 00:15:00 Section 03: The 6 Key Leadership Principle Introduction to the 6 Principles of Great Managers 00:02:00 Principle 1. They Bridge Knowledge Gaps 00:03:00 Principle 2. They Give Clear Direction 00:02:00 Principle 3. They Prioritise Effectively 00:02:00 Principle 4. They Set the Team Up For Success 00:04:00 Principle 5. They Establish Themselves Strategically 00:06:00 Principle 6. They Build Great Networks 00:05:00 Downloadable Resource: Real-World Application Exercise Book 00:55:00 Certificate and Transcript Order Your Certificates or Transcripts 00:00:00

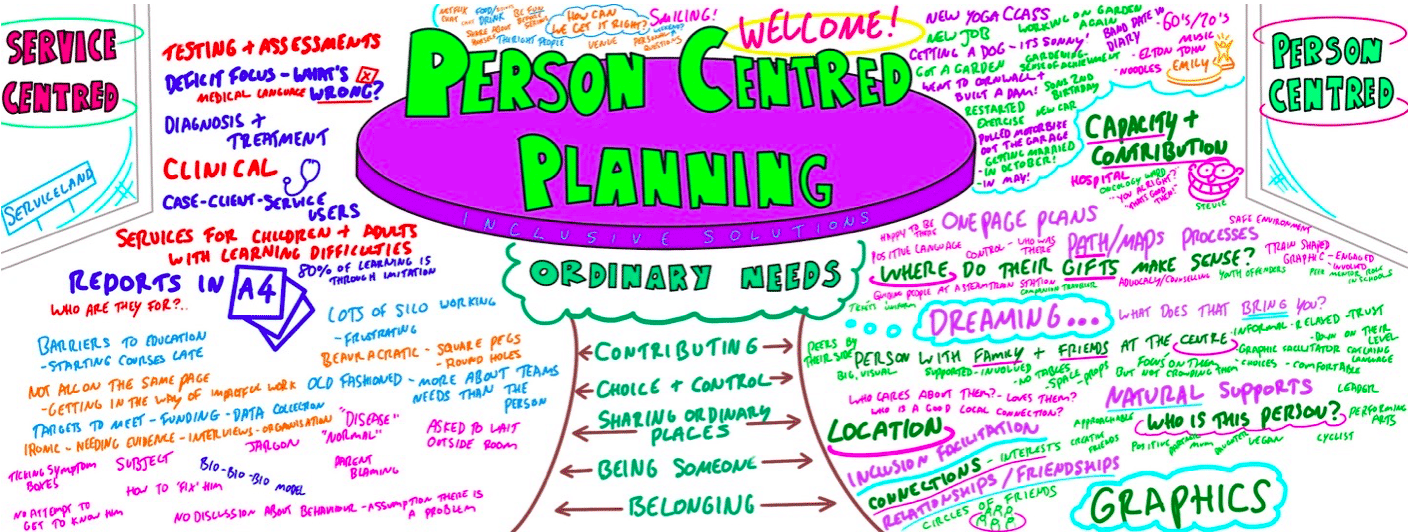
Person Centred Planning using PATH and MAPs
By Inclusive Solutions
What is Person Centred Planning? How is it different to any other kind of meeting or planning? On this day all will become clear… Give your team the opportunity to pause and reflect on what matters most to them about the work they do. The act of listening to each other creates relationship and strengthens trust and inclusion within the team – in creating a shared vision, groups of people build a sense of commitment together. They develop images of ‘the future we want to create together’, along with the values that will be important in getting there and the goals they want to see achieved along the way. Unfortunately, many people still think ’vision’ is the top leader’s job. In schools, the ‘vision task’ usually falls to the Headteacher and/or the governors or it comes in a glossy document from the local authority or the DfES. But visions based on authority are not sustainable. Making inclusive action plans using full participation and graphic facilitation Drawing on the planning tools MAPS and PATH (Pearpoint, Forest and O’Brien 1997) and other facilitation sources we use both process and graphic facilitation to enable the group to build their picture of what they would love to see happening within their organisation/community in the future and we encourage this to be a positive naming, not just a list of the things they want to avoid. Jack Pearpoint, Marsha Forest and John O’Brien developed these innovative approaches in North America and they are being used successfully in many parts of the UK. The planning can focus on an individual, group or organisation and provides a powerful problem solving opportunity, which is flexible and robust enough for many occasions. Tell the story, find the dream, touch the nightmare, and explore who you are, what are the gifts and strengths of the person or group, what are the needs of those present and what is the action plan for the future? Learning objectives Participants understand Person Centred Planning and its values and applications Participants have skills and confidence to facilitate PATH/MAP processes Participants learn graphic as well as process facilitation skills Strengthens practitioners inclusive practice Provides additional tools for those involved in inclusive work in schools and the community Further develop problem solving and planning skills Course Content The course answers the questions: Need to find new ways to bring Pathway Planning alive? Bored with annual reviews, transition plans and review meetings? Want to find a way of making meetings and planning feel more real and engaging? Need an approach, which engages a young person respectfully together with his or her family and friends? Want the ultimate visual record of the process of a meeting, which will help everyone, keep track? Want to problem solve and plan for the future of a small or large group, service or organisation up to the size of an LA? Inclusive Solutions offer an introductory day to person centred planning or a 3 – 10 session course which is practical as well as values based. Participants will receive direct individualised coaching and training. We will cover: The person being at the centre Family members and friends being full partners Planning reflecting the person’s capacities, what is important to the person and specifying the support they require to make a full contribution to their community Planning building a shared commitment to action that will uphold the person’s rights Planning leading to continual listening, learning and action and helping the person get what they want out of life. Essential Lifestyle Planning, PATH MAPS Personal Futures Planning.
Restorative Justice
By Inclusive Solutions
Restorative Interventions in Schools A “Restorative Solution” is a non-adversarial approach to conflict resolution where the person who has done something wrong in a given situation becomes accountable to those s/he has harmed. This person is then given the opportunity to “make up” for their inappropriate behaviour through agreement and reparation. An intervention can involve a formal conference, or it can be a simple conversation on a corridor or playground. Restorative Interventions work with all ages of young people, and the techniques can be used in parental meetings, can prevent exclusions and challenge poor behaviour. Our feedback tells us that when problems between young people are addressed in this way, those problems rarely reoccur. Course Category Behaviour and Relationships Teaching and Learning Description Restorative Interventions in Schools A “Restorative Solution” is a non-adversarial approach to conflict resolution where the person who has done something wrong in a given situation becomes accountable to those s/he has harmed. This person is then given the opportunity to “make up” for their inappropriate behaviour through agreement and reparation. An intervention can involve a formal conference, or it can be a simple conversation on a corridor or playground. Restorative Interventions work with all ages of young people, and the techniques can be used in parental meetings, can prevent exclusions and challenge poor behaviour. Our feedback tells us that when problems between young people are addressed in this way, those problems rarely reoccur. Also Available on line– self paced learning… give it a try! Testimonials ‘Interesting. A different approach. Another ‘weapon’ in the armoury. Will try to use.’ ‘Made me think how I can approach confrontations differently. Useful, reminded me where my focus should be and that things will be difficult at times, but that’s no excuse to stay in the comfort zone!’ ‘Excellent day. Lots of ideas to take away and use on a daily basis. Thanks.’ ‘Nice to do some work on behaviour without sanctions and restraints in mind’ Learning Objectives To introduce participants to Restorative Interventions To develop understanding of value and role in developing inclusive practice of Restorative Interventions To develop and practise Restorative Intervention skills processes Opportunity to reflect on professional practice Mutual support established with other ‘front line’ practitioners Who Is It For ? Multi Agency Teams Social workers CAMHS teams Year Managers Primary and secondary staff Early Years and School based Practitioners Heads and Deputies SENCOs Advanced Skills Teachers Primary and secondary teachers Local Authority Support Services Course Content This can be introduced in one day or as a workshop but is better offered over a series of sessions. The course answers the questions: What should we do if rewards and punishments do not work? Can we find an alternative process to recommending permanent exclusion or special unit or school placement? Struggling with a child for whom praise and sanctions seem ineffective? Want to develop a more restorative school or team? I need a process which works with relationships not just behaviour. What will work for our relationships policy? This day’s training will enable participants to facilitate Short Restorative Conferences, and will suggest ways of working with young people and others that will help to repair broken relationships, and challenge some difficult behaviour. Including all those affected by an incident in its resolution is a powerful way of producing significant improvements in behaviour. We will cover: What are restorative solutions? Background and detailed teaching of processes Inclusion values underpinning this work Processes modelled and opportunities to try process out Practical setting up of restorative conferences Stories and outcomes

PERSON CENTRED PLANNING TRAINING
By Inclusive Solutions
Person Centred Planning (PCP) is a powerful tool for visioning, future planning and team building. It gets everyone on the same page and creates a progressive, constructive atmosphere so it is especially useful for people who are feeling ‘stuck’ or in a really desperate situation. The PCP processes we teach are called “PATH” and “MAPs”, and are both used for different situations. “To facilitate a group, family, team or organisation in thinking together around a given challenge or issue. Here is an opportunity to experience for real the person centred, futures planning tool – MAP/PATH” (Pearpoint, Forest, O’Brien. 1989). PCP can focus on an individual, with family and friends in the room supporting them, or focus on a group who want to set goals, and realise their potential as a team. PCP focuses on hopes and dreams, so is a very positive approach to planning, and utilises graphic facilitation, making it a very friendly way of working that is accessible to everyone. This unique, hands on PCP training course explores the difference between ‘person-centred thinking’ and ‘service-centred thinking’. Traditionally, we have not always listened deeply enough to the needs of those we are planning for. Often ‘medical model’ thinking dominates our planning for those with the most complex needs. Typically we plan ‘about’ rather than ‘with’ children and adults. We examine this ‘service centred’ approach that most professionals are used to, and question its limitations. This values based approach to inclusion will give participants the ‘theory’ behind why thinking and working in a person centred way is so important, and challenges people to strive for more for the people we are planning around. In addition to the knowledge and theory, this course empowers participants to facilitate their own Person Centred Plans using the PATH or MAPs process and provides the skills to do this effectively. Learning objectives Strengthen understanding of how to think in a person centred way Knowledge of the steps of PATH / MAPs process Ability to facilitate PATH/MAPs process Ability to graphically facilitate a PCP meeting Develop problem solving and planning skills Inclusive Solutions offer: We could provide training for a team of staff, or we could facilitate a PATH or MAP around a disabled person. We can also offer bespoke training packages, please enquire for more details. 1 day “Introduction to PCP” with up to 100 attendees focusing on ‘Person Centred Thinking’ – includes live demonstration of PATH or MAPs Process (for a member of the group / with a student and their family/friends). 2 day “PCP Skills training” – More skills focused, lots of practice. Includes Graphics academy, Process academy and Coaching (Best with under 30 attendees). 2 day “Introduction to PCP with Skills training” – includes live demonstration of PATH or MAPs process, Graphics academy, Process academy and Coaching (Best with under 30 attendees). Half day PATH or MAP for a disabled person, led by two experienced Inclusive Solutions facilitators. Full day PATH or MAP for a team, organisation or group such as a full school staff led by two experienced Inclusive Solutions facilitators. Typical Structure of 2-Day “Introduction to PCP with Skills” Training This course is most useful when delivered over 2 days so we have time to cover the ‘skills’ behind facilitating a PATH or a MAP. Here is an example of what usually happens. If you need us to, we can tailor any of our courses to suit your specific needs. Day 1 AM What makes a good welcome?Service centred thinking and working – what does it involve, how does it make people feel?Reflections on current practices – what is useful and what is harmful?Introduction to underpinning values of person centred thinking and working In depth exploration of the fundamentals of inclusion – encouraging identity, focusing on gifts and capacities Sharing success stories, and personal insight from experienced psychologistsShowcasing a number of practical PCP process in actionIntroduction to the “5 service accomplishments”, or “5 ordinary needs” PM Full PATH process demonstration, led by 2 experienced facilitators – one process facilitator and one graphic facilitatorVolunteers will make notes on what they see the facilitators doing and feedback at the end of the dayFeedback, Q&A Day 2 AM Graphics Academy – we will ease you into the world of graphic facilitation and show you how simple it really is – includes live coaching and graphics tutorial, then participants will practice on each other by facilitating the first 1 or 2 sections of the PATH Process Academy – we will give you some pointers about holding the group, and facilitating in an inclusive way, this is another chance to practice your new graphics skills PM The group is divided up, and volunteers are selected to facilitate PATH meetings The group then run the PATH’s simultaneously with an experienced coaches in the room to guide and to be available for questionsWe reconvene to feedback about the process and digest all we have learntBrief Q&A session and then final reflections

BRCGS Sécurité des Denrées Alimentaires Issue 9 | Auditeur Principal (5 jours)
5.0(15)By ASK SONIA LTD
Formation officielle Auditeur Principal (Lead Auditor) BRCGS Food v9 (Norme Mondiale pour la Sécurité des Denrées Alimentaires version 9) en français. Dispensée en ligne (Zoom) en direct par un partenaire de formation agréé BRCGS. Frais d'examen et de certificat inclus dans le prix.
