- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
25539 Management courses in Montrose delivered Online
Join our eLearning program with live Zoom training on March 15th and 16th, 2025, to explore how hypnotherapy supports women's health. This workshop focuses on natural fertility, menopause management, and menstrual cycle relief. Learn techniques to reduce anxiety, alleviate symptoms like hot flashes and insomnia, and promote emotional well-being. Empower yourself with holistic tools for enhanced vitality at every stage of life.

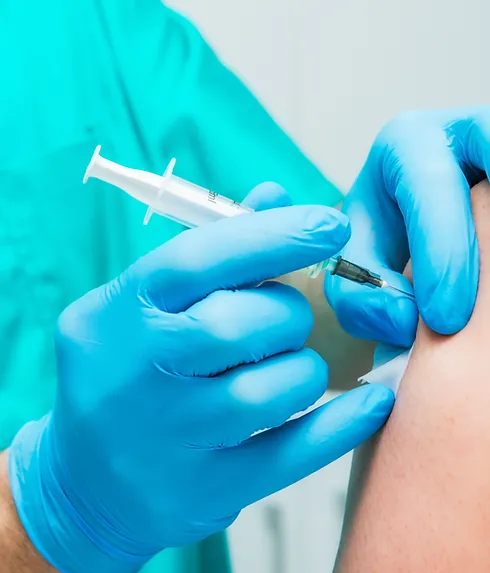
Learn how to administer vaccines or injections ... Nationally Recognised Qualification Includes IM, ID and Sub-Cut Injection methods OCN Accredited - Level 4 (Foundation Degree - FDSc) Covers all steps to safely perform a vaccination Use same techniques and skills for aesthetic therapies Includes B12, Vitamin C and other treatments Essential qualification for all injections Basic understanding of English language required OPEN TO ALL APPLICANTS
The ‘Mastering Management Fundamentals Open Programme’ is a 5 module online course that is designed to improve your management success and performance. It will give you the practical tools and insight to be able to make changes and directly improve your management skills over a 5-6 month period.

Site Management Safety Training Scheme (SMSTS) 5 day course
By SMC Safety Solutions
This five-day course is a must for anyone who is considering or already working in a role with site manager responsibilities. This course covers all relevant legislation affecting safe working in the building, construction, and civil engineering industries. It is endorsed by Build UK as the standard training for all construction managers. Aims To give a clear understanding of health, safety, welfare, and environmental legislation that affects your management role. It highlights the need for risk assessment in the workplace, the implementation of the necessary control measures and adequate communication to sustain a health and safety culture within the workforce. Course Content To give a clear understanding of health, safety, welfare, and environmental legislation that affects your management role. It highlights the need for risk assessment in the workplace, the implementation of the necessary control measures and adequate communication to sustain a health and safety culture within the workforce. Course Content • Health, safety, welfare and environmental legislation affecting your daily work • New guidance and industry best practice • Duties and responsibilities with regards to health, safety, welfare, and the environment • Safe working Prerequisites This course is for you if you’re considering, or already have management responsibilities for planning, organising, monitoring, controlling and administering groups of staff e.g. site manager. Assessment At the end of this course, all delegates will have a clear understanding of controlling health and safety on site from a manager’s role. Certificate The certification for this course is valid for 5 years and is endorsed by Build UK as a standard training for all site managers. To remain certified in this area, you will need to take a refresher course before the expiry date on your certificate, otherwise the full course will need to be retaken. Instructions Please note all online Site Management Safety Training Scheme courses with the venue “Online” will be delivered by a tutor over a video call. This training will be delivered and assessed in English language; therefore, a good standard is required to complete the course. Further attendee information will be sent in a separate email, please check your inbox.

The Real Estate Analyst course has been taught non-stop to global real estate firms over the last 25 years, and is without doubt the core financial modelling training in your career portfolio. Whether you have an upcoming financial modelling test for a new job or an APC exam, the Real Estate Analyst course is the choice for you.

Endometriosis Masterclass
By CCMIG
Endometriosis masterclass. Two day practical theory and hands on course on all aspects of endometriosis management. Expert faculty and live surgical cases.

Good Laboratory Practice Refresher and Hot Topics
By Research Quality Association
Course Information Join us for a comprehensive refresher focusing on crucial Good Laboratory Practice (GLP) requirements, including an emphasis on data integrity, recent developments, and emerging trends gleaned from MHRA inspections. The programme dives into specific domains such as risk assessment, OECD guidance on sponsor influence, and the advisory from OECD on QA. Additionally, delegates can benefit from a dedicated GLP clinic, facilitating discussions on understanding and upholding GLP compliance. Is this course for you? This course is tailored for study directors, principal investigators, test facility management, and QA professionals seeking to refresh their knowledge and responsibilities within the GLP framework. Tutors Tutors will be comprised of (click the photos for biographies): Vanessa Grant -, - Tim Stiles Consultant, Qualogy Ltd Programme Please note timings may be subject to alteration. Day 1 09:00 Registration, Welcome and Introduction 09:20 Development of Good Laboratory Practice A reminder of the history of GLP, its current scope and application, with a synopsis of current UK, European and international standards. 09:50 Roles and Responsibilities of Study Director, Test Facility Management, Principal Investigator, Test Site Management, Study Staff and QA A reminder of the roles and responsibilities with regard to the GLP management and oversight of the Test Facility and the management and control of the study, as defined by GLP. 10:30 Break 10:45 Workshop 1 Workshop 1 Roles and responsibilities 11:15 Influence of Sponsors The published OECD Position Paper No. 21 regarding Possible Influence of Sponsors on conclusions of GLP Studies is reviewed and discussed. 11:45 Data Integrity The fundamentals of data integrity according to the OECD Guidance No. 22 on Data Integrity is discussed along with the responsibilities of Study Director, Test Facility Management, and study staff in ensuring the integrity of the GLP study data. 12:30 Lunch 13:15 Quality Assurance and GLP OECD Advisory No. 23 (Revision of OECD No.4)- A walk through of the changes to the OECD Guidance on the role and activities of Quality Assurance 13:45 Quality Improvement Tools and GLP The tools that might be considered for GLP and their role and operation when used in Test Facilities- OECD Position Paper No.24 published July 2022 14:15 Workshop 2 Workshop 2 Change control 14:30 Risk Assessment How should we assess risk and how can we use the process to assist in evaluation audit findings? 15:00 Break 15:15 Current hot topics in GLP Explore the current issues in Industry and trends /types of Regulatory inspection findings 15:50 GLP Clinic An opportunity to discuss any other issues regarding understanding and maintaining GLP Compliance. 16:30 Close of Course Extra Information Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 7 Points Development Level Learn



