- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
3881 Link courses
Quality Systems for Research Laboratories
By Research Quality Association
Course Information This highly interactive course will provide guidance on why and how to implement a quality system successfully into the research laboratory. By doing so, you will position your innovation for the success it deserves. But leave things as they are and there is a good chance that your science will not realise its full potential should success, and its consequences, come your way. A quality system in your research laboratory is the most effective and efficient way to: Help scientists work more efficiently Ensure discoveries can be defended Protect the value of intellectual property. This course is particularly aimed at those working in early phase research environments which are not constrained by the regulatory requirements of the Good Practice regulations but are producing intellectual property, testing and/or products for the therapeutic market. For organisational reasons, rather than regulatory ones, this is a place where you need to get it right. The programme is delivered by leaders in the field who, quite simply, ‘have done it’. Whether delegates are at senior management level seeking strategic direction, a laboratory head wishing to deliver science that will stand the test of time or a quality professional thrown in at the deep end, this course will provide key insight and practical guidance to underpin future success. Based on risk based systems, tried and tested over many years in the workplace, the programme will help delegates to define, train, implement and monitor the quality of their research, irrespective of field or discipline. Delegates will learn how to help position their organisation for success. Course content: Delegates will be guided thoughtfully through each key component of the process in a stimulating learning environment. The course probes all avenues of the research quality arena, from an initial understanding of the cultural aspects of the scientific discovery environment, to managing quality in outsourced research programmes. Computer systems and e-data security in the research environment will be discussed and pragmatic solutions described to help manage the ballooning cloud of e-data. In addition, the ever blurring boundary between the regulated and non-regulated research environments will be discussed and delegates given perspective on future developments in the field. With this knowledge, delegates will be able to get it ‘right first time’. Is this course for you? The course is designed for all those involved in the research laboratory quality arena and it has been tailored to meet the needs of scientific management, bench scientists and quality professionals alike. Delegates get immediate access to highly experienced tutors who will share their wisdom and insights in an area where few others have been successful. The course is linked with the RQA guidance which builds on years of experience and forms the foundation of the programme. Tutors Tutors will be comprised of (click the photos for biographies): Louise Handy Director, Handy Consulting Ltd Sandrine Bongiovanni Associate Director in Research and Quality Compliance, Novartis Programme Please note timings may be subject to alteration. Day 1 09:00 Registration 09:10 Welcome and Introductions 09:20 History and Overview of the Field Examples of business and regulatory risks and the consequences of low quality in research. A look at the standards and guidelines that exist. 10:00 The Culture, the Politics and the Scientist's Perspective Understanding research environments, the drivers and the challenges. 10:30 Break 10:45 Workshop - Risk Management Thinking about risk management and prioritisation. Looking at the critical factors for the implementations of a successful quality system. 12:15 Workshop - Feedback 12:45 Lunch 13:45 Personnel, Plans, Procedures, Facilities, Equipment, Materials and Reagents Looking at planning the work, defining procedures in a way which promotes robust science without compromising brilliance and ensuring that all these elements are demonstrably fit for their intended purpose. 14:30 Workshop - Assay Validation How much validation is required at what stage? What do we need to validate an assay? 15:00 Workshop - Feedback 15:15 Research, Work Records, Archives and Research Review Data and records which are accurate, attributable, legally attestable and safe to permit reconstruction experiments and studies. Looking at aspects of the work where there is a chance to review, correct or improve the science, the data and the processes. 16:15 Continual Improvement and Quality Systems Reviewing implementation of a quality system, finding opportunities for improvement, understanding culture change. 16:45 Questions and Answers 17:00 Close of Course Extra Information Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 7 Points Development Level Develop

Tailwind CSS From Scratch - Learn by Building Projects
By Packt
This is a fun project-based course to learn how to create awesome layouts using the Tailwind CSS framework and to get comfortable using utility classes over something like Bootstrap, which uses component-based classes. Experiment and learn all about certain aspects of Tailwind with the help of this course.

Good Clinical Practice Auditing - Principles and Practice
By Research Quality Association
Course Information Our comprehensive course is used as a gateway to those stepping into the world of auditing clinical studies. Tailored for those already acquainted with Good Clinical Practice (GCP) and those transitioning from other audit disciplines, this programme stands as a pivotal guide. Pre-existing knowledge of GCP will significantly enhance your learning experience in auditing against these guidelines. How is this course run? Engage in immersive workshops providing hands-on practice with auditing techniques in a GCP context. Our seasoned tutors, boasting extensive audit experience, intertwine theory with practical insights drawn from their own professional journeys. What will I learn? A comprehensive understanding of the historical backdrop and objectives driving Good Clinical Practice, incorporating the latest industry developments Solid grounding in quality assurance activities aligned with regulatory standards Insight into potential pitfalls within clinical trials and the pivotal role of auditors in addressing these issues Clarity on the roles and responsibilities inherent to clinical trials auditing Exposure to a diverse range of audit techniques complemented by illustrative examples and supportive documents A nuanced understanding of regulatory inspectors' activities Expanded professional networks to propel your auditing career forward. Benefits include: A clear understanding of the role of the auditor under Good Clinical Practice improved audits Improved Good Clinical Practice compliance for your clinical trials. This course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Examine particular aspects of Good Clinical Practice. Tutors Tutors will be comprised of (click the photos for biographies): Rosemarie Corrigan EVP Global Quality, Worldwide Clinical Trials Cathy Dove Director and Owner, Dove Quality Solutions Julie Kelly Associate Director, Clinical Quality Assurance, Corcept Therapeutics Susana Tavares Director of Research Quality Assurance, - Programme Please note timings may be subject to alteration. Day 1 12:30 Registration 13:00 Welcome and Objectives for the first day of the course 13:30 Laying the Foundations Introduction to the clinical development process, the concepts of quality assurance, quality control and audit. 14:30 Break 15:00 Patient Protection Requirements for informed consent and ethics committee. Access to source documentation. Including a patient protection exercise. 16:05 Workshop 1 - Case Study on Informed Consent 16:45 End of Day Questions and Answers 17:00 Close of Day Day 2 08:50 Questions and Answers from Day 1 09:00 Effective Site Audits The procedures involved in selecting and setting up audits at investigator sites. 09:40 Workshop 1 - Planning the Effective Audit 10:30 Break 10:45 Source Data Verification The need for and purpose of verifying data. 11:25 Workshop 2 - Source Data Verification 12:30 Lunch 13:30 IMP Management The requirements surrounding the distribution of investigational medicinal products. Accountability from release to destruction. 14:15 Critical Document Audits The conduct of other study specific audits including protocols, databases and reports. 15:00 Break 15:15 Non-compliance Determining the acceptability of data. 16:00 Fraud - Fact or Fiction? How to identify fraud and its consequences 16:45 End of Day Questions and Answers 17:00 Close of Day Day 3 08:50 Questions and Answers from Days 1 and 2 09:00 Auditing Third Parties A review of audits of contract research organisations. 10:00 System Audits The concept of auditing processes across many clinical trials, including a practical exercise in process mapping. 10:45 Break 11:00 Workshop 3 - Process Mapping 11:45 Effective Audits Where theory meets reality. 12:30 Lunch 13:20 Audit Reports - Closing the Loop An examination of the processes which follow the evidence gathering phase of the audit. 14:20 Workshop 4 - Audit Reports Audit reports, corrective and preventive action. 15:00 Break 15:10 Regulatory Inspection Auditors and regulatory inspections -how the QA team can help the organisation to perform during a regulatory inspection. 15:55 Final Questions and Answers 16:10 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 17 Points Development Level Develop

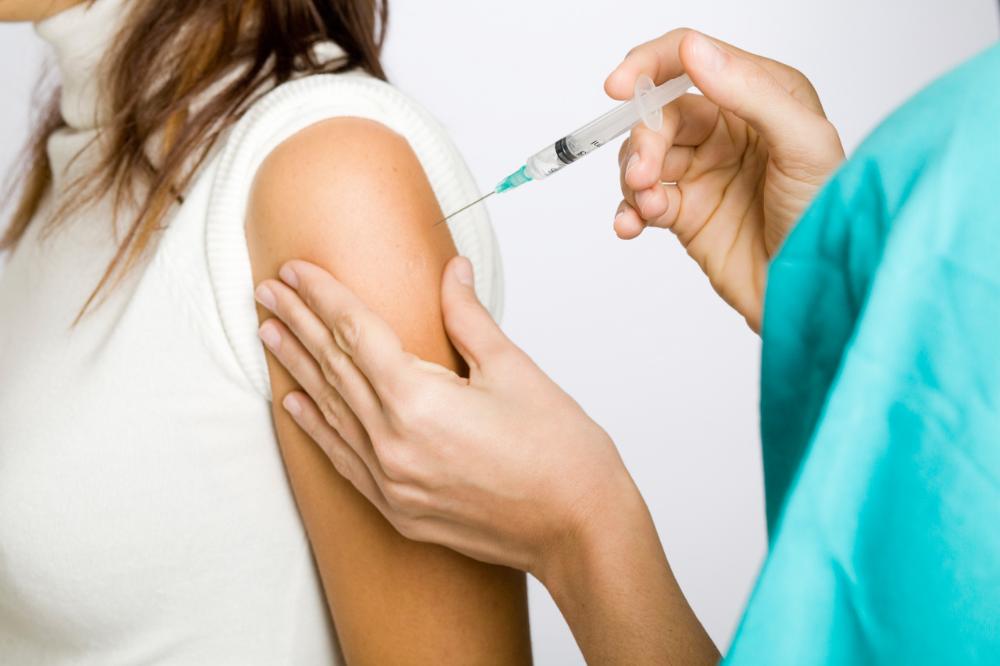
Update your vaccination / injection certificate online ... Update and renew your Nationally Recognised Qualification - dual accreditation Awards a new current certificate and updates your registration OCN Accredited - Level 4 (foundation degree - FDSc) CPD Accredited Add a further 3 FDSc level credits to your C.V. Includes IM, ID and Sub-Cut Injection methods Complete Part 1 (theory) only to renew your registration Add an optional training kit and full video instructions Include practical training at home with the purchase of our comprehensive Practise@Home Training kit with instructional video tutorial for only £39.95. Add this option at time of booking. ONLY OPEN TO APPLICANTS WHO HAVE PREVIOUSLY COMPLETED GEOPACE VACCINATION COURSE
Rhino 3d and V-ray for Rhino Basic to Intermediate Training
By London Design Training Courses
Why Learn Rhino 3d and V-ray for Rhino Basic to Intermediate Training Course? Course Link Learn Rhino 3D and V-Ray for Rhino Basic to Intermediate Training Course. Designed for beginners and experienced users, this course empowers you with essential modeling and rendering techniques. Choose between in-person or live online sessions. Duration: 16 hrs. Method: 1-on-1, Personalized attention. Schedule: Tailor your own schedule by pre-booking hours of your choice, available from Mon to Sat between 9 am and 7 pm. London Design Training Course Certified tutors for Rhino 3D and Vray Training - Online, On-Site, or In-Class one to one. Choose from a diverse range of Rhino courses conducted by expert trainers with extensive production experience. Rhino 3D and V-Ray Comprehensive Training Course Duration: 16 hours Course Overview: Learn Rhino 3D and V-Ray with our Basic to Intermediate Training Course. Perfect for beginners, this course equips you with the skills to navigate Rhino's interface, create captivating 3D models, apply textures, and utilize V-Ray for stunning renders. Course Outline: I. Introduction to Rhino 3D and V-Ray (1 hour) Get familiar with Rhino 3D and V-Ray interfaces and essential tools Master viewport navigation with expert controls Understand units and tolerance for precise designs Customize your workspace for seamless workflow II. Creating Basic 3D Objects (3 hours) Craft captivating 3D shapes and objects Edit objects and work with curves for refined designs Explore surfaces and solids for advanced modeling Utilize transformations and copying techniques for enhancements III. Materials and Textures (2 hours) Unleash the power of materials and textures on your designs Apply stunning materials to bring your creations to life Enhance your designs with custom materials for an aesthetic boost IV. Basic Lighting Techniques in V-Ray (2 hours) Discover the magic of lighting with V-Ray for impactful renders Utilize V-Ray lights and HDRI maps for striking lighting effects Adjust lighting settings for impeccable results V. Enhancing Renders with V-Ray Settings (3 hours) Optimize V-Ray render settings for the best output Adjust global illumination and reflection settings for desired looks Elevate renders with dynamic effects like depth of field and motion blur VI. Advanced Modeling Techniques (2 hours) Master advanced modeling tools for intricate shapes Refine curve and surface creation and editing skills Handle solids with Boolean operations and trimming techniques VII. Advanced Lighting Techniques in V-Ray (2 hours) Create and adjust artificial lights for perfect illumination Utilize V-Ray's Sun and Sky system for realism Craft custom HDR images for enhanced lighting VIII. Exporting and Importing (1 hour) Learn file formats for seamless sharing and collaboration Prepare models for 3D printing with best practices Integrate models from other software for a unified workflow IX. Conclusion and Next Steps (30 minutes) Recap course knowledge and highlights Access valuable tips and resources for continuous learning Engage in interactive Q&A for feedback and support Resources: Explore V-Ray with a free trial at: https://www.chaosgroup.com/vray/sketchup/free-trial Access material library at: https://www.vray-materials.de/ Discover textures at: https://textures.com/ Downloads: Download Rhino 7 for Windows for a full 90-day evaluation at: https://www.rhino3d.com Experience Rhino 7 for Mac Evaluation with a user-friendly interface and seamless performance for 90 days. Upon completing the Rhino 3D and V-Ray Basic to Intermediate Training Course, participants can expect to achieve the following learning outcomes: Proficiency in Rhino 3D and V-Ray: Develop a comprehensive understanding of Rhino 3D and V-Ray interfaces, tools, and functionalities, enabling participants to navigate and utilize the software effectively. 3D Modeling Skills: Master the art of creating captivating 3D objects and shapes using Rhino 3D, including editing, transforming, and refining designs for diverse applications. Material Application: Acquire the ability to apply and customize materials and textures to enhance the visual appeal and realism of 3D models, elevating the quality of designs. Lighting Techniques: Gain expertise in utilizing V-Ray lighting tools, such as V-Ray lights and HDRI maps, to achieve striking lighting effects in rendered scenes. Rendering Excellence: Learn to optimize V-Ray render settings, control global illumination, and apply dynamic effects like depth of field and motion blur for high-quality and impressive renders. Advanced Modeling: Develop skills in advanced modeling techniques, including handling curves, surfaces, and solids, enabling participants to tackle complex design challenges. Lighting Mastery: Acquire the knowledge and expertise to create and adjust artificial lights, utilize the Sun and Sky system, and craft custom HDR images for realistic lighting setups. Seamless File Management: Understand various file formats for exporting and importing 3D models, facilitating efficient collaboration and preparation for 3D printing. Creative Problem-Solving: Enhance creative thinking and problem-solving abilities through hands-on practice and exploration of diverse design scenarios. Professional Presentation: Learn post-production techniques in Photoshop to enhance renders and create visually appealing design presentations for effective communication. Continuous Learning: Access valuable resources and tips to encourage continuous learning and professional growth in Rhino 3D and V-Ray.

Microsoft Office 2016 Complete Bundle Course (Word, Excel, PowerPoint, Outlook and Access)
4.3(43)By John Academy
Description: Microsoft Office 2016 Complete Bundle Course (Word, Excel, PowerPoint, Outlook and Access) is intended to prepare you to get complete control on Microsoft Office 2016. It is designed to educate about Microsoft Office 2016. In this course, you will learn about the basic features of Microsoft Word such as creating a new document, personalizing the Microsoft Word interface, adding graphics, etc. Besides, you will learn about the techniques of controlling page appearance, proofing and editing a document. Following that, you will learn about the essentials to create and work with an electronic spreadsheet. You will be able to insert and delete text or images, adjust cells, create columns, rows and much more. This advanced course helps you to navigate the PowerPoint environment, create and save PowerPoint presentation, delivering advanced text editing etc. Finally, you will learn how to set up and use Outlook on Windows. It will assist you in creating and sending messages, managing contacts, using the calendar, managing tasks, working with notes and so much more. Overall, this course is created especially for you to provide all information to access Microsoft Office 2016. Enroll today and get benefit from this course. Learning Outcomes: Changing user information, sharing documents, working with comments and comparing document changes Collaborate on documents by reviewing them, merging changes and co-authoring Discover how to best use reference tools, like captions, cross-references, bookmarks, hyperlinks, footnotes, endnotes, citations and bibliographies Working with master documents and subdocuments Adding cover page; creating an index, table of contents, and ancillary tables; and managing outlines to simplify and manage large documents Suppressing information from a document, setting editing restrictions, adding digital signature platforms, and restricting document access Find out techniques for saving and exporting form data, along with creating and manipulating forms Using SharePoint Server with Word in order to create, compare and combine different document versions Ways to update worksheet properties, create and edit macros, applying conditional formatting and add data validation criteria Auditing worksheets through tracing cells, troubleshooting invalid data and formula errors, watching and evaluating formulas, and creating a data list online Create Sparklines, scenarios and interactive data using Power View Perform what-if analysis and statistical analysis with the Analysis ToolPak Ways to multitask by consolidating data, linking cells in different workbooks, and merging them Export Excel data, import delimited text files, integrate Excel data with the web and create web queries How to import and export XML data PowerPoint environment and how to modify it according to needs Customizing user interface and setting up options Create and manage sections, modify slide masters and slide layouts, add headers and footers, modify notes master and handout master Creating and modifying SmartArt Adding audio video to presentations and customizing animations and transitions to enhance presentation Better collaboration by adding comments, reviews, storing and sharing presentations on the web Setting up and creating custom slide shows, adding hyperlink and action buttons, and recording a presentation Security and distribution of presentations through various outlets Change message settings, properties, options, using automatic replies and inserting objects Sort and filter options, organising and searching messages, managing junk mail and mailbox Managing advanced calendar options, additional calendars, meeting responses, and assigning tasks Advanced, forward and export contact options, handling contacts and editing electronic business cards Sharing calendars and contacts whilst also delegating access to mail folders to others Modifying data file settings and backing up Outlook items Email security and configuring email message security Designing forms through adding and setting controls, creating subforms, using tab pages to organize information, improve navigation, formatting and applying conditional formatting Data, field, form and record validation Creating macros, restricting records using conditions, validate data, automate data entry and convert a macro to VBA in order to enhance user interface design Link tables to external data sources, manage database, determine object dependency, document database, and analyze database performance Allow multiple user access to database by splitting it, implement security, set passwords, convert Access database to ACCDE file, and add digital signatures Create and modify switchboards and startup options Assessment: At the end of the course, you will be required to sit for an online MCQ test. Your test will be assessed automatically and immediately. You will instantly know whether you have been successful or not. Before sitting for your final exam you will have the opportunity to test your proficiency with a mock exam. Certification: After completing and passing the course successfully, you will be able to obtain an Accredited Certificate of Achievement. Certificates can be obtained either in hard copy at a cost of £39 or in PDF format at a cost of £24. Who is this Course for? Microsoft Office 2016 Complete Bundle Course (Word, Excel, PowerPoint, Outlook and Access) is certified by CPD Qualifications Standards and CiQ. This makes it perfect for anyone trying to learn potential professional skills. As there is no experience and qualification required for this course, it is available for all students from any academic background. Requirements Our Microsoft Office 2016 Complete Bundle Course (Word, Excel, PowerPoint, Outlook and Access) is fully compatible with any kind of device. Whether you are using Windows computer, Mac, smartphones or tablets, you will get the same experience while learning. Besides that, you will be able to access the course with any kind of internet connection from anywhere at any time without any kind of limitation. Career Path After completing this course you will be able to build up accurate knowledge and skills with proper confidence to enrich yourself and brighten up your career in the relevant job market. Microsoft Word 2016 Modify User Information 00:15:00 Share a Document 00:30:00 Work with Comments 00:15:00 Compare Document Changes 00:15:00 Review a Document 00:15:00 Merge Document Changes 00:15:00 Coauthor Documents 00:15:00 Add Captions 00:15:00 Add Cross-References 00:15:00 Add Bookmarks 00:15:00 Add Hyperlinks 00:15:00 Insert Footnotes and Endnotes 00:15:00 Add Citations 00:30:00 Insert a Bibliography 00:15:00 Insert Blank and Cover Pages 00:15:00 Insert an Index 00:30:00 Insert a Table of Contents 00:30:00 Insert an Ancillary Table 00:15:00 Manage Outlines 00:30:00 Create a Master Document 00:30:00 Suppress Information 00:30:00 Set Editing Restrictions 00:30:00 Add a Digital Signature to a Document 00:15:00 Restrict Document Access 00:15:00 Create Forms 00:30:00 Manipulate Forms 00:15:00 Form Data Conversion 00:15:00 Create a New Document Version 00:30:00 Compare Document Versions 00:15:00 Merge Document Versions 00:15:00 Activities - Microsoft Word 2016 Advanced 00:00:00 Microsoft Excel 2016 Update Workbook Properties 00:15:00 Activity-Update Workbook Properties 00:05:00 Create and Edit a Macro 00:15:00 Activity-Create and Edit a Macro 00:05:00 Apply Conditional Formatting 00:15:00 Activity-Apply Conditional Formatting 00:05:00 Add Data Validation Criteria 00:15:00 Activity-Add Data Validation Criteriaty 00:05:00 Trace Cells 00:15:00 Activity-Trace Cells 00:05:00 Troubleshoot Invalid Data and Formula Errors 00:15:00 Activity-Troubleshoot Invalid Data and Formula Errors 00:05:00 Watch and Evaluate Formulas 00:15:00 Activity-Watch and Evaluate Formulas 00:05:00 Create a Data List Outline 00:15:00 Activity-Create a Data List Outline 00:05:00 Create Sparklines 00:15:00 Activity_Create Sparklines 00:05:00 Create Scenarios 00:15:00 Activity-Create Scenarios 00:05:00 Perform a What-If Analysis 00:15:00 Activity-Perform a What-If Analysis 00:05:00 Perform a Statistical Analysis with the Analysis ToolPak 00:15:00 Activity-Perform a Statistical Analysis with the Analysis ToolPak 00:05:00 Create Interactive Data with Power View 00:15:00 Activity-Create Interactive Data with Power View 00:05:00 Consolidate Data 00:15:00 Activity-Consolidate Data 00:05:00 Link Cells in Different Workbooks 00:15:00 Activity-Link Cells in Different Workbooks 00:05:00 Merge Workbooks 00:15:00 Activity-Merge Workbooks 00:05:00 Export Excel Data 00:15:00 Activity-Export Excel Data 00:05:00 Import a Delimited Text File 00:14:00 Activity- Import a Delimited Text File 00:05:00 Integrate Excel Data with the Web 00:15:00 Activity-Integrate Excel Data with the Web 00:05:00 Create a Web Query 00:15:00 Activity-Create a Web Query 00:05:00 Import and Export XML Data 00:15:00 Activity-Import and Export XML Data 00:05:00 Activities and Exercise Files - Microsoft Excel 2016 Advanced 00:00:00 Microsoft PowerPoint 2016 Customize the User Interface 01:30:00 Set PowerPoint 2016 Options 00:45:00 Create and Manage Sections 01:00:00 Modify Slide Masters and Slide Layouts 01:15:00 Add Headers and Footers 00:30:00 Modify the Notes Master and the Handout Master 00:45:00 Create SmartArt 00:45:00 Modify SmartArt 01:00:00 Add Audio to a Presentation 00:45:00 Add Video to a Presentation 01:00:00 Customize Animations and Transitions 01:00:00 Add Comments to a Presentation 00:45:00 Review a Presentation 00:45:00 Store and Share Presentations on the Web 01:30:00 Annotate a Presentation 00:45:00 Set Up a Slide Show 00:45:00 Create a Custom Slide Show 00:30:00 Add Hyperlinks and Action Buttons 00:45:00 Record a Presentation 00:15:00 Secure a Presentation 01:30:00 Present a Slide Show Online 01:00:00 Create a Video or a CD 00:45:00 Activities - Microsoft PowerPoint 2016 Advanced 00:00:00 Microsoft Outlook 2016 Insert Advanced Characters and Objects 01:30:00 Modify Message Settings, Properties, and Options 01:30:00 Use Automatic Replies 01:30:00 Sort Messages 00:45:00 Filter Messages 01:00:00 Organize Messages 02:00:00 Search Messages 01:30:00 Manage Junk Mail 01:00:00 Manage Your Mailbox 02:00:00 Manage Advanced Calendar Options 00:45:00 Manage Additional Calendars 01:00:00 Manage Meeting Responses 00:45:00 Assign and Manage Tasks 01:00:00 Edit an Electronic Business Card 01:00:00 Manage Advanced Contact Options 01:30:00 Forward Contacts 00:30:00 Export Contacts 01:00:00 Delegate Access to Mail Folders 01:00:00 Share Your Calendar 00:30:00 Share Your Contacts 00:15:00 Back Up Outlook Items 00:45:00 Change Data File Settings 00:45:00 Configure E-mail Message Security Settings 00:30:00 Activities - Microsoft Outlook 2016 Advanced 00:00:00 Microsoft Access 2016 Add Controls to Forms 01:00:00 Set Form Controls 01:00:00 Create Subforms 00:30:00 Organize Information with Tab Pages 00:30:00 Enhance Navigation with Forms 00:30:00 Format a Form 01:00:00 Apply Conditional Formatting 00:30:00 Field Validation 00:30:00 Form and Record Validation 00:30:00 Create a Macro 01:00:00 Restrict Records Using a Condition 00:30:00 Validate Data Using a Macro 00:30:00 Automate Data Entry Using a Macro 00:30:00 Convert a Macro to VBA 00:30:00 Link Tables to External Data Sources 00:30:00 Manage a Database 00:30:00 Determine Object Dependency 00:30:00 Document a Database 00:30:00 Analyze the Performance of a Database 00:30:00 Split a Database for Multiple User Access 00:30:00 Implement Security 00:30:00 Set Passwords 00:30:00 Convert an Access Database to an ACCDE File 00:30:00 Package a Database with a Digital Signature 00:30:00 Create a Database Switchboard 01:00:00 Modify a Database Switchboard 00:30:00 Set Startup Options 00:30:00 Activities - Microsoft Access 2016 Advanced 00:00:00 Mock Exam Mock Exam- Microsoft Office 2016 Advanced 00:30:00 Final Exam Final Exam- Microsoft Office 2016 Advanced 00:30:00 Order Your Certificates and Transcripts Order Your Certificates and Transcripts 00:00:00

UCLH Paediatric Infectious Diseases Training 26/9/24
By ULCH Paediatric ID
The Paediatric Infectious Diseases team at University College London Hospital are hosting this training for the networks of people who care for Unaccompanied Asylum Seeking Children: Support/ Key workers, foster carers, social workers and members of Looked After Children teams. The aim of the training is to inform you why we carry out infectious screening, how our service works, and how you can support our service. Cademy will send you a link for the Teams meeting once you have registered. Please disseminate these events in your networks. For more information and queries please contact Joanna Martin, Specialist Nurse: joanna.martin8@nhs.net.

UCLH Paediatric Infectious Diseases Training 23/1/25
By ULCH Paediatric ID
The Paediatric Infectious Diseases team at University College London Hospital are hosting this training for the networks of people who care for Unaccompanied Asylum Seeking Children: Support/ Key workers, foster carers, social workers and members of Looked After Children teams. The aim of the training is to inform you why we carry out infectious screening, how our service works, and how you can support our service. Cademy will send you a link for the Teams meeting once you have registered. Please disseminate these events in your networks. For more information and queries please contact Joanna Martin, Specialist Nurse: joanna.martin8@nhs.net.

UCLH Paediatric Infectious Diseases Training
By ULCH Paediatric ID
The Paediatric Infectious Diseases team at University College London Hospital are hosting this training for the networks of people who care for Unaccompanied Asylum Seeking Children: Support/ Key workers, foster carers, social workers and members of Looked After Children teams. The aim of the training is to inform you why we carry out infectious screening, how our service works, and how you can support our service. Cademy will send you a link for the Teams meeting once you have registered. Please disseminate these events in your networks. For more information and queries please contact Joanna Martin, Specialist Nurse: joanna.martin8@nhs.net.

Professional Certificate Course in Introduction to Requirements Management in London 2024
4.9(261)By Metropolitan School of Business & Management UK
This Professional Certificate Course in Introduction to Requirements Management provides a comprehensive overview of fundamental concepts, including the definition and significance of Requirements Management. Participants will gain insights into the intricacies of requirement management processes, understand the critical importance of effective stakeholder management in gathering requirements, and explore the relationship between requirement gathering and strategic project planning. Through targeted modules, learners will also identify key elements of high-quality requirements, grasp diverse types of requirements in project management, and appreciate the pivotal role of requirement management in ensuring project success. After the successful completion of the course, you will be able to learn about the following: Definition and Explanation of Requirements Management Explain the process of requirement management. Explain importance of requirement management Identify the key elements of a good requirement Discuss the different types of requirements in project management. Explain the importance of stakeholder management in requirement gathering Understand the relationship between requirement gathering and project planning This Professional Certificate Course in Introduction to Requirements Management offers a comprehensive understanding of the fundamentals, encompassing the definition and importance of Requirements Management, key elements of effective requirements, diverse requirement types, stakeholder management significance, and the integral connection between requirement gathering and project planning. Participants will gain essential insights into the entire requirement management process, ensuring a solid foundation for successful project implementation. This Professional Certificate Course in Introduction to Requirements Management provides a comprehensive foundation, covering the definition and process of requirements management, emphasizing the importance of stakeholder engagement, and equipping participants with the skills to identify key elements of good requirements, discuss various types, and understand the pivotal link between requirement gathering and effective project planning. Course Structure and Assessment Guidelines Watch this video to gain further insight. Navigating the MSBM Study Portal Watch this video to gain further insight. Interacting with Lectures/Learning Components Watch this video to gain further insight. Introduction to Requirement Management Self-paced pre-recorded learning content on this topic. Introduction To Requirements Management Put your knowledge to the test with this quiz. Read each question carefully and choose the response that you feel is correct. All MSBM courses are accredited by the relevant partners and awarding bodies. Please refer to MSBM accreditation in about us for more details. There are no strict entry requirements for this course. Work experience will be added advantage to understanding the content of the course.The certificate is designed to enhance the learner's knowledge in the field. This certificate is for everyone eager to know more and get updated on current ideas in their respective field. We recommend this certificate for the following audience, Project Managers Business Analysts Software Developers System Analysts Quality Assurance Professionals Product Managers Stakeholders involved in Project Planning Anyone Seeking a Foundation in Requirements Management Average Completion Time 2 Weeks Accreditation 3 CPD Hours Level Advanced Start Time Anytime 100% Online Study online with ease. Unlimited Access 24/7 unlimited access with pre-recorded lectures. Low Fees Our fees are low and easy to pay online.

Search By Location
- Link Courses in London
- Link Courses in Birmingham
- Link Courses in Glasgow
- Link Courses in Liverpool
- Link Courses in Bristol
- Link Courses in Manchester
- Link Courses in Sheffield
- Link Courses in Leeds
- Link Courses in Edinburgh
- Link Courses in Leicester
- Link Courses in Coventry
- Link Courses in Bradford
- Link Courses in Cardiff
- Link Courses in Belfast
- Link Courses in Nottingham