- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
1 Courses in Birmingham
Water Chemistry for Thermal Power Station Plant Chemist & Boiler Engineers
By EnergyEdge - Training for a Sustainable Energy Future
About this Training Course This is an advanced chemistry training course for power plant chemists and boiler engineers wishing to expand their knowledge and skills, and to become more effective in their day-to-day roles dealing with thermal power plant chemistry. This 5 full-day course will provide ample opportunity for robust technical discussion and expand on advanced concepts in thermal power plant cycle chemistry. It focuses only on the steam/water aspects of the thermal power cycle. This course is a MUST for all power plant chemists and boiler engineers. It is also beneficial for anyone involved in power plant operation and maintenance because it provides guidelines and rules for improving power plant performance and reliability. Training Objectives Gain a significant increase in understanding of cycle chemistry in steam power plants and the inter-relationships between plant operation, cycle chemistry and potential failure modes due to corrosion and/or deposition throughout the cycle Gain a thorough understanding of all causes of corrosion in a steam power plant and all the methods used to reduce the corrosion rate in a steam power plant Become better equipped to effectively manage the corrosion and deposition risks in a thermal power plant Learn how to reduce failure rate in boilers and steam power plants and improve plant performance Understand condensate polishing and treatment of condensate return to industrial boilers Discover the causes of boiler water contamination and treatment programs Learn about layup and offline corrosion protection Understand water chemistry limits to prevent steam contamination by carryover Learn about boiler water chemistry guidelines and control of steam chemistry Understand high-purity make-up treatment methods Perform demineralizer calculations Perform system design calculations Gain a thorough understanding of mixed bed polishing and reverse osmosis Target Audience Power Plant Chemists Boiler Engineers Engineers involved in the operation and maintenance of power plants Managers Technicians Maintenance personnel Other technical individuals (this seminar is suitable for individuals who do not have a background in chemical engineering) Course Level Advanced Training Methods Your specialist course leader relies on a highly interactive training method to enhance the learning process. This method ensures that all participants gain a complete understanding of all topics covered. The training environment is highly stimulating, challenging, and effective because the participants will learn by case studies which will allow them to apply the material taught to their own organization. Each delegate will receive a copy of the following materials written by the instructor: 'POWER GENERATION HANDBOOK' second edition, published by McGraw-Hill in 2012 in New York (800 pages) Water Chemistry for Thermal Power Plant Chemists and Boiler Engineers Manual (650 pages) Trainer Your specialist course leader has more than 32 years of practical engineering experience with Ontario Power Generation (OPG), one of the largest electric utility in North America. He was previously involved in research on power generation equipment with Atomic Energy of Canada Limited at their Chalk River and Whiteshell Nuclear Research Laboratories. While working at OPG, he acted as a Training Manager, Engineering Supervisor, System Responsible Engineer and Design Engineer. During the period of time, he worked as a Field Engineer and Design Engineer, he was responsible for the operation, maintenance, diagnostics, and testing of gas turbines, steam turbines, generators, motors, transformers, inverters, valves, pumps, compressors, instrumentation and control systems. Further, his responsibilities included designing, engineering, diagnosing equipment problems and recommending solutions to repair deficiencies and improve system performance, supervising engineers, setting up preventive maintenance programs, writing Operating and Design Manuals, and commissioning new equipment. Later, he worked as the manager of a section dedicated to providing training for the staff at the power stations. The training provided by him covered in detail the various equipment and systems used in power stations. In addition, he has taught courses and seminars to more than four thousand working engineers and professionals around the world, specifically Europe and North America. He has been consistently ranked as 'Excellent' or 'Very Good' by the delegates who attended his seminars and lectures. He written 5 books for working engineers from which 3 have been published by McGraw-Hill, New York. Below is a list of the books authored by him; Power Generation Handbook: Gas Turbines, Steam Power Plants, Co-generation, and Combined Cycles, second edition, (800 pages), McGraw-Hill, New York, October 2011. Electrical Equipment Handbook (600 pages), McGraw-Hill, New York, March 2003. Power Plant Equipment Operation and Maintenance Guide (800 pages), McGraw-Hill, New York, January 2012. Industrial Instrumentation and Modern Control Systems (400 pages), Custom Publishing, University of Toronto, University of Toronto Custom Publishing (1999). Industrial Equipment (600 pages), Custom Publishing, University of Toronto, University of Toronto, University of Toronto Custom Publishing (1999). Furthermore, he has received the following awards: The first 'Excellence in Teaching' award offered by PowerEdge, Singapore, in December 2016 The first 'Excellence in Teaching' award offered by the Professional Development Center at University of Toronto (May, 1996). The 'Excellence in Teaching Award' in April 2007 offered by TUV Akademie (TUV Akademie is one of the largest Professional Development centre in world, it is based in Germany and the United Arab Emirates, and provides engineering training to engineers and managers across Europe and the Middle East). Awarded graduation 'With Distinction' from Dalhousie University when completed Bachelor of Engineering degree (1983). Lastly, he was awarded his Bachelor of Engineering Degree 'with distinction' from Dalhousie University, Halifax, Nova Scotia, Canada. He also received a Master of Applied Science in Engineering (M.A.Sc.) from the University of Ottawa, Canada. He is also a member of the Association of Professional Engineers in the province of Ontario, Canada. POST TRAINING COACHING SUPPORT (OPTIONAL) To further optimise your learning experience from our courses, we also offer individualized 'One to One' coaching support for 2 hours post training. We can help improve your competence in your chosen area of interest, based on your learning needs and available hours. This is a great opportunity to improve your capability and confidence in a particular area of expertise. It will be delivered over a secure video conference call by one of our senior trainers. They will work with you to create a tailor-made coaching program that will help you achieve your goals faster. Request for further information post training support and fees applicable Accreditions And Affliations

Online Options
Show all 21Steps to Purity: Defeating Lust One Step at a Time
By Vince Warner
How to overcome lust and live a life of purity.

VAL05: Equipment Cleaning Validation
By Zenosis
Manufacturers of medicines and healthcare products must establish, validate and maintain an equipment cleaning programme. This is a regulatory requirement because validated cleaning procedures contribute to the assurance of product purity and safety. This module provides a comprehensive account of equipment cleaning validation requirements and procedures. It follows the work of a pharmaceutical company's validation team as they establish and validate the cleaning program for a new production line.
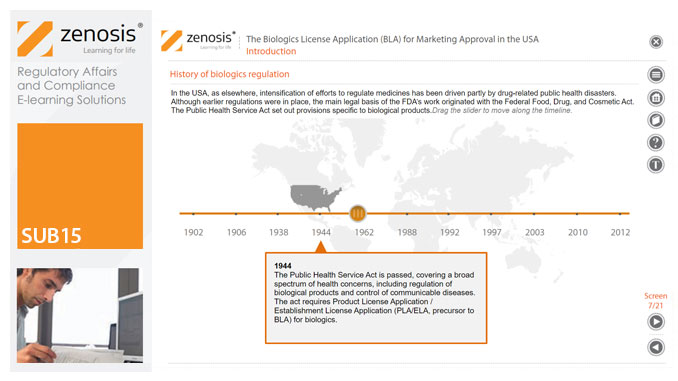
SUB15: The Biologics License Application (BLA) for Marketing Approval in the USA
By Zenosis
This module describes the requirements that must be met to obtain licensure of a biological product. Subjects covered include the regulatory context, the content and format of the BLA submission, the review process, and provisions for expedited development and review.
Complete Practical LINQ Tutorial in C#
By Packt
Master LINQ to entities, LINQ to objects, LINQ to XML, functional programming relying on LINQ

Food Safety : Food Hygiene
By Training Tale
Food Hygiene and Safety refers to a set of food manufacturing techniques aimed at reducing biological food hazards through safe and clean operations in order to protect public health from foodborne diseases. Food safety is a management system used by the food industry to help make sure that hazards are kept to an acceptable level. This Food Safety : Food Hygiene course will teach you how to maintain good hygiene practices among employees and sanitary practices at production sites, both of which are critical factors in preventing food contamination. If you work as a manager or supervisor in the catering industry, this course is perfect for you. It will assist you in exploring daily core responsibilities, such as implementing the fundamentals of a food safety management system. This Food Safety : Food Hygiene course covers all of the most popular topics to help you advance in the professional world and be a better fit for your chosen Food purity & Safety career. As a result of the Food purity & Safety program, your professional development will improve. As you may be aware, effective Food purity and Safety skills are critical because they develop professionalism and generate trust in the workplace. After completing our Food Hygiene & Safety training, you will be more productive and successful. Learning Outcomes After completing this Food Safety : Food Hygiene course successfully, you will be able to: Obtain a thorough understanding of food hygiene and safety precautions. Validate a thorough understanding of the law and regulations governing food safety in the United Kingdom. Determine the risks and hazards of food storage, transportation, and planning. Implement and maintain a strong food safety system at work. Maintain a clean workplace, practice good personal hygiene, and set a high standard for all employees. Demonstrate excellent knowledge of food temperature control. Describe the food safety control system in the workplace. The Food Safety : Food Hygiene course, on the other hand, covers all of the most recent topics in order to bring you up to speed with the most recent job market developments and make you a better match for your chosen vocation. Your skill sets will improve as a result of taking this excellent Food Hygiene and Safety course, which covers topics ranging from basic to advanced. The Food Safety : Food Hygiene course was created by experts with people looking for work and generated as part in mind. Your career will benefit from the Food hygiene & Safety Course. Professionalism and trust in the workplace are fueled by effective food hygiene and safety. Why Choose This Food Safety : Food Hygiene Course from Us Self-paced course, access available from anywhere. Easy to understand, high-quality study materials. This Course developed by industry experts. MCQ quiz after each module to assess your learning. Automated and instant assessment results. 24/7 support via live chat, phone call or email. Free PDF certificate as soon as completing the course. Others Included in this Food Safety : Food Hygiene Course Free One PDF Certificate Access to Content - Lifetime Exam Fee - Totally Free Unlimited Retake Exam [ Note: Free PDF certificate as soon as completing the Food Safety : Food Hygiene course ] This comprehensive Food Safety : Food Hygiene course will provide learners with an in-depth knowledge of best food sterility practices to ensure their business is ranked at a national five-star level. Enrol yourself on this course and learn how to conduct food safety, efficiently train your employees, and implement and track a proper food safety system at work that reduces food danger hazards, controls contamination, and maintains a high standard of hygiene. By completing this food hygiene and safety course, you will get Learn what is expected of food businesses and food handlers in terms of maintaining high food sterility & safety standards. Understand food sterility & safety laws to avoid illness, fines, and possible jail time. Ability to maintain good food hygiene and ensure the safety of employees and customers To summarise, taking this Food Safety : Food Hygiene course is a great way to advance your career. So, what are you holding out for? Enrol in this course immediately! Detailed course curriculum of the Food Safety : Food Hygiene Course: Module 1: Introduction to Food Hygiene General Principles of Food Hygiene Food Hygiene for Product Quality and Safety Personal Hygiene Hygiene and Training of Personnel Module 2: Food Safety- An Introduction Basic Concepts of Food Safety Importance of Food Safety The Principal Responsibility of Operators in Ensuring Food Safety Module 3: Food Hygiene and Safety Legislation in the UK The Food Safety Act (1990) Codes of Practice Food Safety (General Food Hygiene) 1995 Food Safety (Temperature) Regulations 1995 Food Standards Agency Module 4: Food Safety Management (HACCP) Origins of HACCP Objectives of HACCP Benefits of Using HACCP The Seven Principles of HACCP The Opportunity of HACCP Module 5: Nature and Origin of Food Contamination Physical Contamination Chemical Contamination Microbial Contamination Allergenic Contamination of Food Cross Contamination Module 6: Controlling Contamination Food Purchasing and Storage Food Preparation and Cooking Food Refrigeration Food Service and Delivery Module 7: Bacterial Food Poisoning Salmonellas Staphylococcus Aureus Bacillus Cereus Botulism Escherichia Coli Module 8: Non-Bacterial Food Poisoning Chemical Food poisoning Poisonous Plants Animals Toxins Viruses Module 9: Storage and Temperature Control of Foods Dry Foods Refrigerated Products Dairy Products Fresh Meats, Poultry, and Seafood Frozen Foods Module 10: Cleaning and Disinfection Chemical Disinfectants Use of Heat Dry Cleaning Cleaning-in-Place (CIP) Foam cleaning Cleaning Minor Equipment Assessment Method After completing each module of this Food Safety : Food Hygiene Course, you will find automated MCQ quizzes. To unlock the next module, you need to complete the quiz task and get at least 60% marks. Once you complete all the modules in this manner, you will be qualified to request your certification. Certification After completing the MCQ/Assignment assessment for this Food Safety : Food Hygiene course, you will be entitled to a Certificate of Completion from Training Tale. It will act as proof of your extensive professional development. The certificate is in PDF format, which is completely free to download. A printed version is also available upon request. It will also be sent to you through a courier for £13.99. Who is this course for? This Food Safety : Food Hygiene course is ideal for: Food Safety Manager Factory Manager or Supervisor Catering Manager Head Chef Senior food retailers Health & Safety Advisor Restaurant Manager Restaurant and Café owners Kitchen Managers Supervisors or Managers of fast-food outlets and takeaways Supervisory food handlers Requirements There are no specific requirements for this Food Safety : Food Hygiene course because it does not require any advanced knowledge or skills. Students who intend to enrol in this course must meet the following requirements: Good command of the English language Must be vivacious and self-driven Basic computer knowledge A minimum of 16 years of age is required Career path This Food Safety : Food Hygiene course will prepare you for a variety of employment choices; here are a few examples: Inspector of Food and Safety, Safety and Hygiene Advisor, Restaurant Manager, Head Chef, Food Safety Manager, Food Safety Officer. Certificates Certificate of completion Digital certificate - Included

Level 2 Food Hygiene & Safety Course
By Training Tale
Food Hygiene and Safety refers to a set of food manufacturing techniques aimed at reducing biological food hazards through safe and clean operations in order to protect public health from foodborne diseases. Food safety is a management system used by the food industry to help make sure that hazards are kept to an acceptable level. This Level 2 Food Hygiene & Safety course will teach you how to maintain good hygiene practices among employees and sanitary practices at production sites, both of which are critical factors in preventing food contamination. If you work as a manager or supervisor in the catering industry, this Level 2 Food Hygiene & Safety course is perfect for you. It will assist you in exploring daily core responsibilities, such as implementing the fundamentals of a food safety management system. This Level 2 Food Hygiene & Safety course covers all of the most popular topics to help you advance in the professional world and be a better fit for your chosen Food purity & Safety career. As a result of the Level 2 Food Hygiene & Safety program, your professional development will improve. As you may be aware, effective Food purity and Safety skills are critical because they develop professionalism and generate trust in the workplace. After completing our Food Hygiene & Safety training, you will be more productive and successful. Learning Outcomes After completing this Level 2 Food Hygiene & Safety course successfully, you will be able to: Obtain a thorough understanding of food hygiene and safety precautions. Validate a thorough understanding of the law and regulations governing food safety in the United Kingdom. Determine the risks and hazards of food storage, transportation, and planning. Implement and maintain a strong food safety system at work. Maintain a clean workplace, practice good personal hygiene, and set a high standard for all employees. Demonstrate excellent knowledge of food temperature control. Describe the food safety control system in the workplace. The Level 2 Food Hygiene & Safety course, on the other hand, covers all of the most recent topics in order to bring you up to speed with the most recent job market developments and make you a better match for your chosen vocation. Your skill sets will improve as a result of taking this excellent Food Hygiene & Safety course, which covers topics ranging from basic to advanced. The Level 2 Food Hygiene & Safety course was created by experts with people looking for work and generated as part in mind. Your career will benefit from the Food hygiene and Safety Course. Professionalism and trust in the workplace are fueled by effective food hygiene and safety. Why Choose Level 2 Food Hygiene & Safety from Us Self-paced course, access available from anywhere. Easy to understand, high-quality study materials. Level 2 Food Hygiene & Safety Course developed by industry experts. MCQ quiz after each module to assess your learning. Automated and instant assessment results. 24/7 support via live chat, phone call or email. Free PDF certificate as soon as completing the Level 2 Food Hygiene & Safety course. Others Included in this Level 2 Food Hygiene & Safety Course Free One PDF Certificate Access to Content - Lifetime Exam Fee - Totally Free Unlimited Retake Exam [ Note: Free PDF certificate as soon as completing the Level 2 Food Hygiene & Safety course ] This comprehensive Level 2 Food Hygiene & Safety course will provide learners with an in-depth knowledge of best food sterility practices to ensure their business is ranked at a national five-star level. Enrol yourself on this Level 2 Food Hygiene & Safety course and learn how to conduct food safety, efficiently train your employees, and implement and track a proper food safety system at work that reduces food danger hazards, controls contamination, and maintains a high standard of hygiene. By completing this Level 2 Food Hygiene & Safety course, you will get Learn what is expected of food businesses and food handlers in terms of maintaining high food sterility & safety standards. Understand food sterility & safety laws to avoid illness, fines, and possible jail time. Ability to maintain good food hygiene and ensure the safety of employees and customers To summarise, taking this Level 2 Food Hygiene & Safety course is a great way to advance your career. So, what are you holding out for? Enrol in this course immediately! Detailed course curriculum of this Course: Module 1: Introduction to Food Hygiene General Principles of Food Hygiene Food Hygiene for Product Quality and Safety Personal Hygiene Hygiene and Training of Personnel Module 2: Food Safety- An Introduction Basic Concepts of Food Safety Importance of Food Safety The Principal Responsibility of Operators in Ensuring Food Safety Module 3: Food Hygiene and Safety Legislation in the UK The Food Safety Act (1990) Codes of Practice Food Safety (General Food Hygiene) 1995 Food Safety (Temperature) Regulations 1995 Food Standards Agency Module 4: Food Safety Management (HACCP) Origins of HACCP Objectives of HACCP Benefits of Using HACCP The Seven Principles of HACCP The Opportunity of HACCP Module 5: Nature and Origin of Food Contamination Physical Contamination Chemical Contamination Microbial Contamination Allergenic Contamination of Food Cross Contamination Module 6: Controlling Contamination Food Purchasing and Storage Food Preparation and Cooking Food Refrigeration Food Service and Delivery Module 7: Bacterial Food Poisoning Salmonellas Staphylococcus Aureus Bacillus Cereus Botulism Escherichia Coli Module 8: Non-Bacterial Food Poisoning Chemical Food poisoning Poisonous Plants Animals Toxins Viruses Module 9: Storage and Temperature Control of Foods Dry Foods Refrigerated Products Dairy Products Fresh Meats, Poultry, and Seafood Frozen Foods Module 10: Cleaning and Disinfection Chemical Disinfectants Use of Heat Dry Cleaning Cleaning-in-Place (CIP) Foam cleaning Cleaning Minor Equipment Assessment Method After completing each module of the Level 2 Food Hygiene & Safety, you will find automated MCQ quizzes. To unlock the next module, you need to complete the quiz task and get at least 60% marks. Once you complete all the modules in this manner, you will be qualified to request your certification. Certification After completing the MCQ/Assignment assessment for this Level 2 Food Hygiene & Safety course, you will be entitled to a Certificate of Completion from Training Tale. It will act as proof of your extensive professional development. The certificate is in PDF format, which is completely free to download. A printed version is also available upon request. It will also be sent to you through a courier for £13.99. Who is this course for? This Level 2 Food Hygiene & Safety course is ideal for: Food Safety Manager Factory Manager or Supervisor Catering Manager Head Chef Senior food retailers Health & Safety Advisor Restaurant Manager Restaurant and Café owners Kitchen Managers Supervisors or Managers of fast-food outlets and takeaways Supervisory food handlers Requirements There are no specific requirements for this Level 2 Food Hygiene & Safety course because it does not require any advanced knowledge or skills. Career path This Level 2 Food Hygiene & Safety course will prepare you for a variety of employment choices; here are a few examples: Inspector of Food and Safety, Safety and Hygiene Advisor, Restaurant Manager, Head Chef, Food Safety Manager, Food Safety Officer. Certificates Certificate of completion Digital certificate - Included

Food : Safety & Hygiene
By Training Tale
Food Hygiene and Safety refers to a set of food manufacturing techniques aimed at reducing biological food hazards through safe and clean operations in order to protect public health from foodborne diseases. Food safety is a management system used by the food industry to help make sure that hazards are kept to an acceptable level. This Food : Safety & Hygiene course will teach you how to maintain good hygiene practices among employees and sanitary practices at production sites, both of which are critical factors in preventing food contamination. If you work as a manager or supervisor in the catering industry, this Food : Safety & Hygiene course is perfect for you. It will assist you in exploring daily core responsibilities, such as implementing the fundamentals of a food safety management system. This Food : Safety & Hygiene course covers all of the most popular topics to help you advance in the professional world and be a better fit for your chosen Food purity & Safety career. As a result of the Food : Safety & Hygiene program, your professional development will improve. As you may be aware, effective Food purity and Safety skills are critical because they develop professionalism and generate trust in the workplace. After completing our Food : Safety & Hygiene training, you will be more productive and successful. After completing this Food : Safety & Hygiene course successfully, you will be able to: Obtain a thorough understanding of food hygiene and safety precautions. Validate a thorough understanding of the law and regulations governing food safety in the United Kingdom. Determine the risks and hazards of food storage, transportation, and planning. Implement and maintain a strong food safety system at work. Maintain a clean workplace, practice good personal hygiene, and set a high standard for all employees. Demonstrate excellent knowledge of food temperature control. Describe the food safety control system in the workplace. The Food : Safety & Hygiene course, on the other hand, covers all of the most recent topics in order to bring you up to speed with the most recent job market developments and make you a better match for your chosen vocation. Your skill sets will improve as a result of taking this excellent Food : Safety & Hygiene course, which covers topics ranging from basic to advanced. Why Choose This Food : Safety & Hygiene from Us Self-paced course, access available from anywhere. Easy to understand, high-quality study materials. Food : Safety & Hygien Course developed by industry experts. MCQ quiz after each module to assess your learning. Automated and instant assessment results. 24/7 support via live chat, phone call or email. Free PDF certificate as soon as completing the Food : Safety & Hygien course. Others Included in this Food : Safety & Hygiene Course Free One PDF Certificate Access to Content - Lifetime Exam Fee - Totally Free Unlimited Retake Exam [ Note: Free PDF certificate as soon as completing the Food : Safety & Hygiene course ] The Food : Safety & Hygiene course was created by experts with people looking for work and generated as part in mind. Your career will benefit from the Food : Safety & Hygiene Course. Professionalism and trust in the workplace are fueled by effective food hygiene and safety. This comprehensive Food : Safety & Hygiene course will provide learners with an in-depth knowledge of best food sterility practices to ensure their business is ranked at a national five-star level. Enrol yourself on this Food : Safety & Hygiene course and learn how to conduct food safety, efficiently train your employees, and implement and track a proper food safety system at work that reduces food danger hazards, controls contamination, and maintains a high standard of hygiene. By completing this Food : Safety & Hygiene course, you will get Learn what is expected of food businesses and food handlers in terms of maintaining high food sterility & safety standards. Understand food sterility & safety laws to avoid illness, fines, and possible jail time. Ability to maintain good food hygiene and ensure the safety of employees and customers To summarise, taking this Food : Safety & Hygiene course is a great way to advance your career. So, what are you holding out for? Enrol in this course immediately! Detailed course curriculum of this Course: *** Food : Safety & Hygiene *** Module 1: Introduction to Food Hygiene General Principles of Food Hygiene Food Hygiene for Product Quality and Safety Personal Hygiene Hygiene and Training of Personnel Module 2: Food Safety- An Introduction Basic Concepts of Food Safety Importance of Food Safety The Principal Responsibility of Operators in Ensuring Food Safety Module 3: Food Hygiene and Safety Legislation in the UK The Food Safety Act (1990) Codes of Practice Food Safety (General Food Hygiene) 1995 Food Safety (Temperature) Regulations 1995 Food Standards Agency Module 4: Food Safety Management (HACCP) Origins of HACCP Objectives of HACCP Benefits of Using HACCP The Seven Principles of HACCP The Opportunity of HACCP Module 5: Nature and Origin of Food Contamination Physical Contamination Chemical Contamination Microbial Contamination Allergenic Contamination of Food Cross Contamination Module 6: Controlling Contamination Food Purchasing and Storage Food Preparation and Cooking Food Refrigeration Food Service and Delivery Module 7: Bacterial Food Poisoning Salmonellas Staphylococcus Aureus Bacillus Cereus Botulism Escherichia Coli Module 8: Non-Bacterial Food Poisoning Chemical Food poisoning Poisonous Plants Animals Toxins Viruses Module 9: Storage and Temperature Control of Foods Dry Foods Refrigerated Products Dairy Products Fresh Meats, Poultry, and Seafood Frozen Foods Module 10: Cleaning and Disinfection Chemical Disinfectants Use of Heat Dry Cleaning Cleaning-in-Place (CIP) Foam cleaning Cleaning Minor Equipment Assessment Method After completing each module of the Food : Safety & Hygiene, you will find automated MCQ quizzes. To unlock the next module, you need to complete the quiz task and get at least 60% marks. Once you complete all the modules in this manner, you will be qualified to request your certification. Certification After completing the MCQ/Assignment assessment for this Food : Safety & Hygiene course, you will be entitled to a Certificate of Completion from Training Tale. It will act as proof of your extensive professional development. The certificate is in PDF format, which is completely free to download. A printed version is also available upon request. It will also be sent to you through a courier for £13.99. Who is this course for? This Food : Safety & Hygiene course is ideal for: Food Safety Manager Factory Manager or Supervisor Catering Manager Head Chef Senior food retailers Health & Safety Advisor Restaurant Manager Restaurant and Café owners Kitchen Managers Supervisors or Managers of fast-food outlets and takeaways Supervisory food handlers Requirements There are no specific requirements for this Food : Safety & Hygiene course because it does not require any advanced knowledge or skills. Students who intend to enrol in this Food : Safety & Hygiene course must meet the following requirements: Good command of the English language Must be vivacious and self-driven Basic computer knowledge A minimum of 16 years of age is required Career path This Food Hygiene and Safety Food : Safety & Hygiene course will prepare you for a variety of employment choices; here are a few examples: Inspector of Food and Safety, Safety and Hygiene Advisor, Restaurant Manager, Head Chef, Food Safety Manager, Food Safety Officer. Certificates Certificate of completion Digital certificate - Included

Food Hygiene and Food Safety
By Training Tale
Food Hygiene and Food Safety refers to a set of food manufacturing techniques aimed at reducing biological food hazards through safe and clean operations in order to protect public health from foodborne diseases. Food Hygiene and Food Safety is a management system used by the food industry to help make sure that hazards are kept to an acceptable level. This Food Hygiene and Food Safety course will teach you how to maintain good hygiene practices among employees and sanitary practices at production sites, both of which are critical factors in preventing food contamination. If you work as a manager or supervisor in the catering industry, this course is perfect for you. It will assist you in exploring daily core responsibilities, such as implementing the fundamentals of a food safety management system. This Food Hygiene and Food Safety course covers all of the most popular topics to help you advance in the professional world and be a better fit for your chosen Food purity & Safety career. As a result of the Food Hygiene and Food Safety program, your professional development will improve. As you may be aware, effective Food Hygiene and Safety skills are critical because they develop professionalism and generate trust in the workplace. After completing our Food Hygiene and Food Safety training, you will be more productive and successful. Learning Outcomes After completing this Food Hygiene and Food Safety course successfully, you will be able to: Obtain a thorough understanding of food hygiene and safety precautions. Validate a thorough understanding of the law and regulations governing food safety in the United Kingdom. Determine the risks and hazards of food storage, transportation, and planning. Implement and maintain a strong food safety system at work. Maintain a clean workplace, practice good personal hygiene, and set a high standard for all employees. Demonstrate excellent knowledge of food temperature control. Describe the food safety control system in the workplace. The Food Hygiene and Food Safety course, on the other hand, covers all of the most recent topics in order to bring you up to speed with the most recent job market developments and make you a better match for your chosen vocation. Your skill sets will improve as a result of taking this excellent Food Hygiene and Food Safety course, which covers topics ranging from basic to advanced. Why Choose This Food Hygiene and Food Safety Course from Us Self-paced course, access available from anywhere. Easy to understand, high-quality study materials. This Food Hygiene and Food Safety course is developed by industry experts. MCQ quiz after each module to assess your learning. Automated and instant assessment results. 24/7 support via live chat, phone call or email. Free PDF certificate as soon as completing the course. Others Included in this Food Hygiene and Food Safety Course Free One PDF Certificate Access to Content - Lifetime Exam Fee - Totally Free Unlimited Retake Exam [ Note: Free PDF certificate as soon as completing this Food Hygiene and Food Safety course ] The Food Hygiene and Food Safety course was created by experts with people looking for work and generated as part in mind. Your career will benefit from the Food purity & Safety Course. Professionalism and trust in the workplace are fueled by effective food hygiene and safety. This comprehensive Food Hygiene and Food Safety course will provide learners with an in-depth knowledge of best food sterility practices to ensure their business is ranked at a national five-star level. Enrol yourself on this course and learn how to conduct food safety, efficiently train your employees, and implement and track a proper food safety system at work that reduces food danger hazards, controls contamination, and maintains a high standard of hygiene. By completing this Food Hygiene and Food Safety course, you will get - Learn what is expected of food businesses and food handlers in terms of maintaining high food sterility & safety standards. Understand food sterility & safety laws to avoid illness, fines, and possible jail time. Ability to maintain good food hygiene and ensure the safety of employees and customers To summarise, taking this Food Hygiene And Safety course is a great way to advance your career. So, what are you holding out for? Enrol in this course immediately! ----------------------------------------------------------------------------- Detailed course curriculum of Food Hygiene and Food Safety Course Module 1: Introduction to Food Hygiene General Principles of Food Hygiene Food Hygiene for Product Quality and Safety Personal Hygiene Hygiene and Training of Personnel Module 2: Food Safety- An Introduction Basic Concepts of Food Safety Importance of Food Safety The Principal Responsibility of Operators in Ensuring Food Safety Module 3: Food Hygiene and Safety Legislation in the UK The Food Safety Act (1990) Codes of Practice Food Safety (General Food Hygiene) 1995 Food Safety (Temperature) Regulations 1995 Food Standards Agency Module 4: Food Hygiene and Food Safety Management (HACCP) Origins of HACCP Objectives of HACCP Benefits of Using HACCP The Seven Principles of HACCP The Opportunity of HACCP Module 5: Nature and Origin of Food Contamination Physical Contamination Chemical Contamination Microbial Contamination Allergenic Contamination of Food Cross Contamination Module 6: Controlling Contamination Food Purchasing and Storage Food Preparation and Cooking Food Refrigeration Food Service and Delivery Module 7: Bacterial Food Poisoning Salmonellas Staphylococcus Aureus Bacillus Cereus Botulism Escherichia Coli Module 8: Non-Bacterial Food Poisoning Chemical Food poisoning Poisonous Plants Animals Toxins Viruses Module 9: Storage and Temperature Control of Foods Dry Foods Refrigerated Products Dairy Products Fresh Meats, Poultry, and Seafood Frozen Foods Module 10: Cleaning and Disinfection Chemical Disinfectants Use of Heat Dry Cleaning Cleaning-in-Place (CIP) Foam cleaning Cleaning Minor Equipment Assessment Method After completing each module of the Food Hygiene and Food Safety, you will find automated MCQ quizzes. To unlock the next module, you need to complete the quiz task and get at least 60% marks. Once you complete all the modules in this manner, you will be qualified to request your certification. Certification After completing the MCQ/Assignment assessment for this Food Hygiene and Food Safety course, you will be entitled to a Certificate of Completion from Training Tale. It will act as proof of your extensive professional development. The certificate is in PDF format, which is completely free to download. A printed version is also available upon request. It will also be sent to you through a courier for £13.99. Who is this course for? This Food Hygiene and Food Safety course is ideal for: Food Safety Manager Factory Manager or Supervisor Catering Manager Head Chef Senior food retailers Health & Safety Advisor Restaurant Manager Restaurant and Café owners Kitchen Managers Supervisors or Managers of fast-food outlets and takeaways Supervisory food handlers Requirements There are no specific requirements for this Food Hygiene and Food Safety course because it does not require any advanced knowledge or skills. Career path This Food Hygiene and Food Safety course will prepare you for a variety of employment choices; here are a few examples: Inspector of Food and Safety, Safety and Hygiene Advisor, Restaurant Manager, Head Chef, Food Safety Manager, Food Safety Officer. Certificates Certificate of completion Digital certificate - Included

With the increasing demand for alternative medicine, herbalists have a steady stream of clients and a good earning potential. Therefore, now is a better time than ever to pursue herbalism as a career. Our Herbalist course will help you establish yourself as a viable herbalist! This Diploma in Master Herbalist at QLS Level 5 course is endorsed by The Quality Licence Scheme and accredited by CPDQS (with 150 CPD points) to make your skill development & career progression more accessible than ever! This online Master Herbalist course is designed to introduce students to the principles and practices of herbalism. Students will learn about the history and traditions of herbalism, as well as the safety and effectiveness of different herbs and herbal remedies. The Master Herbalist course will cover a variety of topics, including plant identification, herb-drug interactions, and the ethical and legal considerations of practising herbalism. So enrol right now! At the end of this Master Herbalist course, you will be able to : Understand the history and traditions of herbalism and the role that plants have played in medicine throughout history. Identify common herbs and understand their properties and uses. Understand the safety and effectiveness of different herbs and herbal remedies, and be able to evaluate the quality and purity of herbal products. Understand the ethical and legal considerations of practising herbalism, including the importance of informed consent and the role of regulation in the field. Use the principles of herbalism to support the health and well-being of individuals and communities. Why Prefer This Herbalist at QLS Level 5 Course? Opportunity to earn a certificate endorsed by the Quality Licence Scheme & another accredited by CPDQS which is completely free. Get a free student ID card! (£10 postal charge will be applicable for international delivery) Innovative and engaging content. Free assessments 24/7 tutor support. Our online Master Herbalist course is designed to provide a comprehensive introduction to herbal medicine. Through a series of interactive modules, you will learn about the history and principles of herbalism, as well as how to identify and use various medicinal plants. You will also learn how to create your own herbal remedies, including tinctures, salves, and teas. By the end of this Master Herbalist course, you will have the knowledge and skills needed to confidently use herbs as a natural form of health care for yourself and others. This Master Herbalist course is suitable for beginners and those interested in pursuing a career as a herbalist. *** Course Curriculum *** Here is the curriculum breakdown of the Herbalist at QLS Level 5 course: Module 01: Basics of Herbal Medicine Module 02: Begin with Botany Module 03: Chemistry of Herbs Module 04: Holy Herbs and Herbs of China Module 05: Preparation of Herbal Remedies Module 06: Herbalist Approach and Conventional Herbal Medicines Module 07: Ayurvedic Medicine Module 08: Aromatherapy Module 09: Herbal Essences for Hair Module 10: Herbal Skin Care Module 11: Creating a Successful Herbal Business Assessment Process You have to complete the assignment questions given at the end of the course and score a minimum of 60% to pass each exam. Our expert trainers will assess your assignment and give you feedback after you submit the assignment. You will be entitled to claim a certificate endorsed by the Quality Licence Scheme after you have completed all of the Diploma in Master Herbalist at QLS Level 5 exams. CPD 150 CPD hours / points Accredited by CPD Quality Standards Who is this course for? This Master Herbalist course will come in handy for: Those interested in natural health and wellness People who want to learn how to use herbs as a form of self-care Aspirants looking to start a career as a herbalist People interested in complementary and alternative medicine Those seeking a more holistic approach to health and wellness. Requirements No prior background or expertise is required. Career path Herbalism is an ancient career which has resurfaced with the technological touch and is gradually expanding. Here are some of the careers that we can help our learners pursue Herbalist - £33,928 annually Herbal Medicine-Maker - Over £35,000 annually Herbal Educator - Over £30,000 annually Holistic Health Practitioner - Around £35,000 annually Herbal Consultant - £33,928 Annually Certificates CPDQS Accredited Certificate Digital certificate - Included Diploma in Master Herbalist at QLS Level 5 Hard copy certificate - Included Show off Your New Skills with a Certificate of Completion After successfully completing the Diploma in Master Herbalist at QLS Level 5, you can order an original hardcopy certificate of achievement endorsed by the Quality Licence Scheme andalso you can order CPDQSAccredited Certificate that is recognised all over the UK and also internationally. The certificates will be home-delivered, completely free of cost.

Search By Location
- purity Courses in London
- purity Courses in Birmingham
- purity Courses in Glasgow
- purity Courses in Liverpool
- purity Courses in Bristol
- purity Courses in Manchester
- purity Courses in Sheffield
- purity Courses in Leeds
- purity Courses in Edinburgh
- purity Courses in Leicester
- purity Courses in Coventry
- purity Courses in Bradford
- purity Courses in Cardiff
- purity Courses in Belfast
- purity Courses in Nottingham