- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
3883 Healthcare courses
Mental Health First Aid - 8 Premium Courses Bundle
By NextGen Learning
Unlock the power of understanding with our comprehensive Mental Health First Aider Training bundle, meticulously designed eight courses that equip you with vital insights into mental health. This Mental Health First Aider Training bundle encompasses eight courses that delve into the heart of mental health awareness. Delve into eight immersive CPD Accredited courses, each a standalone course: Course 01: Mental Health First Aid Certification Course 02: Mental Health Nursing Course 03: Concussion and Brain Injury Awareness Course 04: Mental Health Support Worker Course 05: Advanced Diploma in Psychology Course 06: Counselling Skills Training Course 07: Substance Abuse & Mental Health Interventions Course 08: Addiction and Mental Health - Dual Diagnosis Explore crucial insights into psychological health, master empathetic communication techniques, and gain proficiency in effective intervention strategies. With a focus on substance abuse, dual diagnosis complexities, and brain injury awareness, this Mental Health First Aider Training bundle equips you to be a reliable pillar of support. Enrol now and make a difference! Learning Outcomes Recognize early signs of distress and provide empathetic support. Communicate effectively with individuals facing mental health challenges. Intervene in cases of substance abuse and dual diagnosis. Understand the impact of concussions and brain injuries. Assist as a proficient Mental Health Support Worker. Utilise psychology and counselling skills to offer guidance and support. The Mental Health First Aider Training bundle responds to the growing need for informed allies to bridge the gap between mental health issues and meaningful assistance. Positioned as an oasis of knowledge, this Mental Health First Aider Training bundle encompasses a series of courses, each tackling crucial aspects of mental health with precision and depth. From mastering empathetic communication to unravelling the intricacies of substance abuse and dual diagnosis scenarios, the courses lay a robust foundation. So "how" can you make a tangible impact? The Mental Health First Aider Training bundle arms you with techniques enabling you to identify signs of distress and offer empathetic support effectively. It's a transformational solution for anyone looking to contribute positively to the lives of those facing mental health challenges. The promise of this Mental Health First Aider Training bundle lies in its potential to equip you with the expertise to be an empathetic pillar of support. By embracing the knowledge gleaned from these courses, you're pledging to be a reliable source of understanding and assistance, empowering individuals on their mental health journeys. Your commitment to learning and compassion will create a ripple effect, fostering a culture of empathy and well-being. CPD 80 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Compassionate supporters. Practical solution seekers. Effective communicators. Substance abuse interveners. Brain injury informers. Future Support Workers. Career path Mental Health Support Worker: £21,000 - £26,000 annually Substance Abuse Counsellor: £23,000 - £30,000 annually Community Outreach Worker: £20,000 - £25,000 annually Mental Health Advocate: £25,000 - £30,000 annually Rehabilitation Counsellor: £24,000 - £32,000 annually Crisis Intervention Specialist: £22,000 - £28,000 annually Social Services Coordinator: £23,000 - £28,000 annually Certificates CPD Certificate Of Completion Digital certificate - Included 8 Digital Certificates Are Included With This Bundle CPD Quality Standard Hardcopy Certificate (FREE UK Delivery) Hard copy certificate - £9.99 Hardcopy Transcript - £9.99

SUB05: Electronic Common Technical Document (eCTD)
By Zenosis
The eCTD is mandatory for all applications for marketing approval and all subsequent related submissions in the European Economic Area, the USA and Canada. Other countries intend to make its use mandatory. The eCTD specification has been developed to facilitate the global electronic submission, review and lifecycle management of medicinal product dossiers for regulatory applications. It broadens the scope of the CTD to include information on variations, renewals and amendments, so that it is no longer a static document but is updatable throughout the life of the product. This module outlines the eCTD specification, discusses the approach to regional differences in dossiers, and provides guidance on creation of an eCTD submission. The module provides a training and reference tool that will be of particular value to those new to the use of the format.

CT09: Good Clinical Practice Inspections and Audits
By Zenosis
The module describes general principles of GCP inspection and audit, discusses preparation for an inspection, and sets out in detail what European and US FDA inspectors will examine. Finally it describes post-inspection actions by the regulator and the inspected party.

The Level 3 Certificate in End of Life Care provides comprehensive training in palliative care, enabling learners to understand and provide compassionate support to individuals at the end of their life. The course covers various aspects of end-of-life care, from advance care planning to legal issues, preparing learners to offer effective and holistic support during this critical stage. Learning Outcomes: Introduce the concepts and principles of palliative and end-of-life care. Identify the different stages of illness and understand their implications for care. Conduct a holistic assessment for palliative care. Familiarize with advance care planning and its significance in end-of-life care. Provide appropriate care to individuals at the end of their life. Understand the processes and considerations related to care after death. Access further support, training, and development resources in end-of-life care. Explore legal issues and ethical considerations in palliative care. Gain familiarity with essential terminology related to end-of-life care. Access additional resources and appendices to enhance knowledge in this field. Certification After studying the course materials of the Level 3 Certificate in End of Life Care you will be able to take the MCQ test that will assess your knowledge. After successfully passing the test you will be able to claim the pdf certificate for £4.99. Original Hard Copy certificates need to be ordered at an additional cost of £8. Who is this course for? This Level 3 Certificate in End of Life Care does not require you to have any prior qualifications or experience. You can just enrol and start learning. Prerequisites This Level 3 Certificate in End of Life Care was made by professionals and it is compatible with all PC's, Mac's, tablets and smartphones. You will be able to access the course from anywhere at any time as long as you have a good enough internet connection. Career path As this course comes with multiple courses included as bonus, you will be able to pursue multiple occupations. This Level 3 Certificate in End of Life Care is a great way for you to gain multiple skills from the comfort of your home. Course Curriculum End of Life Care Module 1: Introduction to Palliative & End of Life Care 00:45:00 Module 2: Identification of the Stage of Illness 00:15:00 Module 3: Palliative Care Holistic Assessment 00:15:00 Module 4: Advance Care Planning 00:30:00 Module 5: Care at the End of Life 00:15:00 Module 6: Care after Death 00:15:00 Module 7: Further Support, Training and Development 00:10:00 Module 8: Legal Issues 00:30:00 Module 9: Glossary of Terms 00:15:00 Module 10: Appendices 00:10:00 Mock Exam Mock Exam - Level 3 Certificate in End of Life Care 00:20:00 Final Exam Final Exam - Level 3 Certificate in End of Life Care 00:20:00

Medical Administration, Clinical Coding and Billing This course teaches you the core skills needed for medical administration, clinical coding, and billing. You will learn how to manage schedules, organise patient records, handle medical files, maintain confidentiality, and use proper codes for billing. The course also explains medical terms, ethics, safety rules, and how technology supports healthcare systems. It is ideal for anyone who wants to work in a medical office or support clinical operations. Course Curriculum Module 01: Introduction To Medical And Clinical Administration Module 02: Organise Schedules Module 03: Maintaining Medical Records Module 04: Effective Filing Systems In Medical Offices Module 05: Confidentiality In A Medical Environment Module 06: Controlling Stocks And Supplies Module 07: Medical Jargon And Terminology Module 08: Maintaining Workplace Safety Module 09: Information Technology In Health Care Module 10: Medical (Clinical) Coding And Billing Module 11: Legal Aspects And Ethics (Learn more about this online course)

Asthma Awareness
By Prima Cura Training
This course aims to raise awareness about the triggers, signs and symptoms, and preventive measures to better manage and support people living with asthma.

Observation Skills for Carers
By IOMH - Institute of Mental Health
Overview of Observation Skills for Carers Join our Observation Skills for Carers course and discover your hidden skills, setting you on a path to success in this area. Get ready to improve your skills and achieve your biggest goals. The Observation Skills for Carers course has everything you need to get a great start in this sector. Improving and moving forward is key to getting ahead personally. The Observation Skills for Carers course is designed to teach you the important stuff quickly and well, helping you to get off to a great start in the field. So, what are you looking for? Enrol now! This Observation Skills for Carers Course will help you to learn: Learn strategies to boost your workplace efficiency. Hone your skills to help you advance your career. Acquire a comprehensive understanding of various topics and tips. Learn in-demand skills that are in high demand among UK employers This course covers the topic you must know to stand against the tough competition. The future is truly yours to seize with this Observation Skills for Carers. Enrol today and complete the course to achieve a certificate that can change your career forever. Details Perks of Learning with IOMH One-To-One Support from a Dedicated Tutor Throughout Your Course. Study Online — Whenever and Wherever You Want. Instant Digital/ PDF Certificate. 100% Money Back Guarantee. 12 Months Access. Process of Evaluation After studying the course, an MCQ exam or assignment will test your skills and knowledge. You have to get a score of 60% to pass the test and get your certificate. Certificate of Achievement Certificate of Completion – Digital / PDF Certificate After completing the Observation Skills for Carers course, you can order your CPD Accredited Digital/ PDF Certificate for £5.99. Certificate of Completion – Hard copy Certificate You can get the CPD Accredited Hard Copy Certificate for £12.99. Shipping Charges: Inside the UK: £3.99 International: £10.99 Who Is This Course for? This Observation Skills for Carers is suitable for anyone aspiring to start a career in relevant field; even if you are new to this and have no prior knowledge, this course is going to be very easy for you to understand. On the other hand, if you are already working in this sector, this course will be a great source of knowledge for you to improve your existing skills and take them to the next level. This course has been developed with maximum flexibility and accessibility, making it ideal for people who don’t have the time to devote to traditional education. Requirements You don’t need any educational qualification or experience to enrol in the Observation Skills for Carers course. Do note: you must be at least 16 years old to enrol. Any internet-connected device, such as a computer, tablet, or smartphone, can access this online course. Career Path The certification and skills you get from this Observation Skills for Carers Course can help you advance your career and gain expertise in several fields, allowing you to apply for high-paying jobs in related sectors.

End of Life Care Training and Comprehensive Care of Palliative
By EduXpress
Overview Our comprehensive course is designed to provide you with the necessary skills and knowledge to excel in your field. Developed by professionals with years of experience, this course is perfect for anyone looking to kick start their career or advance their skills. It features an audio-visual presentation and easy-to-learn modules, allowing for a self-paced learning experience. Our expert online support team is available during weekdays to assist you throughout the course. Learning Outcomes Upon completion of the course, you will be able to: Understand the fundamentals and its applications. Develop the necessary skills to succeed in the field . Apply your newly acquired knowledge to real-world scenarios. Create effective and efficient solutions for relevant topics. Enhance your employability and career prospects. It is designed to provide you with the knowledge needed to stand out in the competitive job market. With lifetime access to course materials and the ability to learn at your own pace, this course is the perfect opportunity to improve your skills and advance your career. You can learn at your own pace, from the comfort of your home, as all course materials are accessible online at any time. Why Choose Us? Lifetime access to course materials Full tutor support is available Monday through Friday for all courses Learn essentials skills at your own pace from the comfort of your home Gain a thorough understanding of the course Access informative video modules taught by expert instructors 24/7 assistance and advice available through email and live chat Study the course on your computer, tablet, or mobile device Improve your chances of gaining professional skills and earning potential by completing the course. Why should you study this course? The course offers a comprehensive introduction to the subject matter and provides a solid foundation for further study. It will also help students to gain knowledge and skills that can be applied in their professional or personal life. Assessment The course includes a series of quizzes that will test your understanding and retention of the material covered in the course. The quizzes will help you to identify areas where you may need further practice, and you will have the opportunity to review the course materials as needed. Upon successfully passing the final quiz, you will be able to order your certificate of achievement. Career Path The course is designed to equip you with the skills and knowledge you need to succeed in the field. After completing the course, you will be able to pursue a variety of career opportunities in different industries. Course Content END OF LIFE CARE TRAINING Module 1: Understanding End-of-Life Care Module 2: Legal and Ethical Aspects of End-of-Life Care Module 3: Identification of the Stage of Illness Module 4: Breaking Bad News & Communication in End-of-Life Care Module 5: Pain and Symptom Management Module 6: Psychosocial, Emotional, and Spiritual Care Module 7: Care in the Last Days of Life Module 8: Child and Young Adult End-of-Life Care Module 9: Hospice and Palliative Care Module 10 Nutrition and Hydration at the End of Life

Anti Social Behaviour (ASB)
By Compliance Central
Anti Social Behaviour (ASB) is a pervasive issue, deeply woven into the societal fabric. Anti Social Behaviour (ASB) refers to actions that cause harm or lack consideration for the well-being of others. Such behaviours can include vandalism, public intoxication, harassment, or any act that disrupts the peace and safety of a community. Anti Social Behaviour (ASB) is a growing problem in the UK, with a significant impact on individuals and communities. As a result, the demand for professionals who can understand and address it is rising. If you're interested in a career that is both challenging and rewarding, then consider taking our Anti Social Behaviour Course. Why would you choose the Anti Social Behaviour (ASB) course from Compliance Central: Lifetime access to Anti Social Behaviour (ASB) course materials Full tutor support is available from Monday to Friday with the Anti Social Behaviour (ASB) course Learn Anti Social Behaviour (ASB) skills at your own pace from the comfort of your home Gain a complete understanding of Anti Social Behaviour (ASB) course Accessible, informative Anti Social Behaviour (ASB) learning modules designed by experts Get 24/7 help or advice from our email and live chat teams with the Anti Social Behaviour (ASB) Study Anti Social Behaviour (ASB) in your own time through your computer, tablet or mobile device A 100% learning satisfaction guarantee with your Anti Social Behaviour (ASB) Course Anti Social Behaviour (ASB) Curriculum Breakdown of the Anti Social Behaviour (ASB) Course Curriculum Breakdown of the Course: Module 01: Overview of Anti-social Behaviour Module 02: Family Influences on Anti-social Behaviour Module 03: Anti-Social Behaviour Order Module 04: Anger and Anti-social Behaviour Module 05: Understanding Antisocial Personality Module 06: Prevention of Anti-social Behaviour Anti social behaviour (ASB) Course Learning Outcomes: Define and classify different anti social behaviours. Understand the impact of family on anti social tendencies. Grasp the fundamentals of the Anti Social Behaviour Order. Recognize the correlation between anger and antisocial acts. Delve into the facets of antisocial personality. Identify and suggest strategies for prevention. Assess the societal implications of unchecked anti-social behaviour. CPD 10 CPD hours / points Accredited by CPD Quality Standards Who is this course for? The Anti Social Behaviour (ASB) course helps aspiring professionals who want to obtain the knowledge and familiarise themselves with the skillsets to pursue a career in Anti Social Behaviour (ASB). It is also great for professionals who are already working in Anti Social Behaviour (ASB) and want to get promoted at work. Requirements To enrol in this Anti Social Behaviour (ASB) course, all you need is a basic understanding of the English Language and an internet connection. Career path The Anti Social Behaviour (ASB) course will enhance your knowledge and improve your confidence in exploring opportunities in various sectors related to Anti Social Behaviour (ASB). ASB Officer: £25,000 to £40,000 per year Community Safety Coordinator: £28,000 to £45,000 per year Youth Support Worker: £20,000 to £30,000 per year Neighbourhood Warden: £22,000 to £35,000 per year Certificates CPD Accredited PDF Certificate Digital certificate - Included CPD Accredited PDF Certificate CPD Accredited Hard Copy Certificate Hard copy certificate - £10.79 CPD Accredited Hard Copy Certificate Delivery Charge: Inside the UK: Free Outside of the UK: £9.99 each

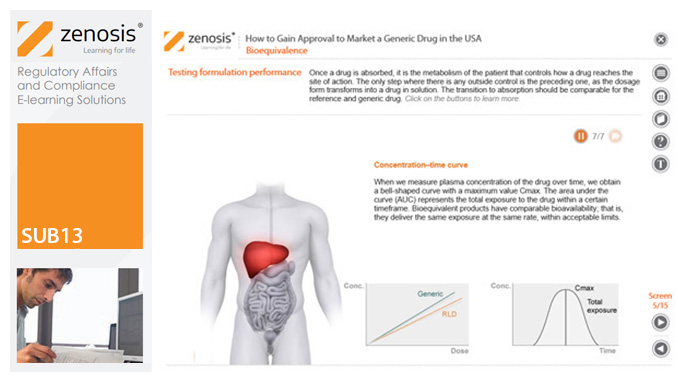
SUB13: How to Gain Approval to Market a Generic Drug in the USA
By Zenosis
This module outlines the legislative and regulatory context for the development of generic drugs and describes the essential role of the Abbreviated New Drug Application (ANDA) in gaining marketing approval. The use of information in the ‘Orange Book’ is explained, as is the role of patent certification in the application. The importance of establishing bioequivalence between a generic and its reference product is emphasised. The module specifies the content and format requirements for an ANDA submission and describes the FDA’s review and approval process. An outline is given of the Generic Drug User Fee Amendments (GDUFA) and the law’s effects on industry players.
Search By Location
- Healthcare Courses in London
- Healthcare Courses in Birmingham
- Healthcare Courses in Glasgow
- Healthcare Courses in Liverpool
- Healthcare Courses in Bristol
- Healthcare Courses in Manchester
- Healthcare Courses in Sheffield
- Healthcare Courses in Leeds
- Healthcare Courses in Edinburgh
- Healthcare Courses in Leicester
- Healthcare Courses in Coventry
- Healthcare Courses in Bradford
- Healthcare Courses in Cardiff
- Healthcare Courses in Belfast
- Healthcare Courses in Nottingham