- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
3860 Healthcare courses
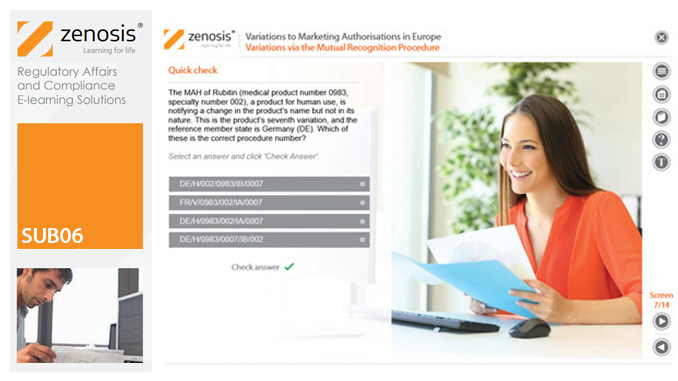
SUB06: Variations to Marketing Authorisations in Europe
By Zenosis
Changes to the terms of marketing authorisations for medicinal products, called variations in Europe, must be notified to or approved by the relevant regulatory authorities. Variations include changes to the composition of products, their manufacturing processes, the way they are used, or the indications for which they are authorised. Common approaches are adopted within the European Economic Area to variations to marketing authorisations approved through the Centralised, Decentralised or Mutual Recognition Procedures. Recent legislation has substantially modified the regulatory requirements and extended them to purely national authorisations by member states. This module, which is fully up to date with the new legislation, covers the classification of variations into their several types and the regulatory requirements, guidance and procedures to be followed for each type.
ESS01: Essentials of EU and US Regulatory Affairs for Human Medicinal Products
By Zenosis
This foundation-level module is the ideal introduction for new entrants to the field of pharmaceutical regulatory affairs and compliance. It describes the principal requirements that must be satisfied to gain and maintain approval to market medicinal products in the USA and Europe. The legal framework and the roles of major players in regulation are presented. The life-cycle of a drug is outlined. The various procedures available for assessment and approval of products are described and their requirements outlined. Obligations to be fulfilled after marketing approval are discussed.

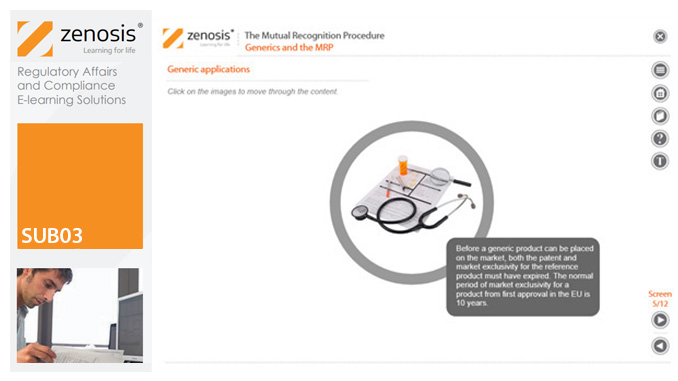
SUB03: The Mutual Recognition Procedure (MRP)
By Zenosis
his module describes the roles of the various players in the procedure, the sequence and duration of the stages involved, and the requirements on content, format and timing of submissions. It discusses the special issues that apply to generic products in the MRP.
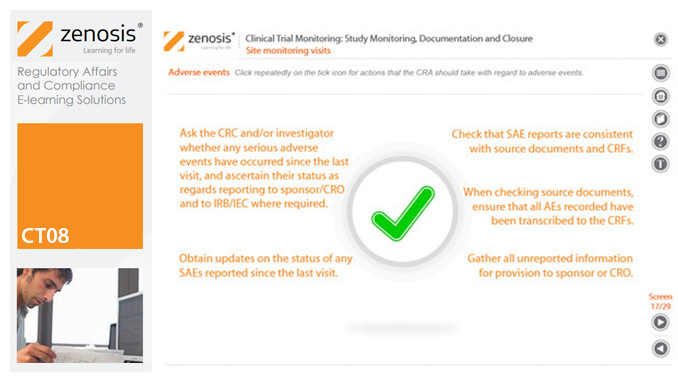
CT08: Clinical Trial Monitoring: Study Monitoring, Documentation and Closure
By Zenosis
The sponsor of a clinical trial must arrange for it to be monitored throughout its duration to ensure that the rights and wellbeing of subjects are protected, the trial data are accurate, complete and verified from source documents, and the conduct of the trial complies with the study protocol, Good Clinical Practice and regulatory requirements. In this module we describe how a Clinical Research Associate (CRA) monitors an ongoing trial to its conclusion.
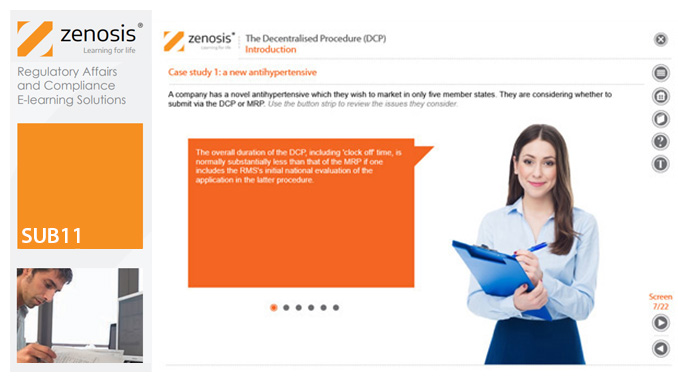
SUB11: The Decentralised Procedure (DCP)
By Zenosis
This module describes the roles of the various players in the procedure, the sequence and duration of the stages involved, and the requirements on content, format and timing of submissions. It discusses the special issues that apply to generic products in the DCP.
Family Law, Family Support Worker and Safeguarding Diploma Transform your career with our Family Law, Family Support Worker and Safeguarding Diploma. Become the Family Support Worker that families trust. Drive change as an informed and skilled Family Support Worker. Learning Outcomes: Master key Family Law principles relevant to a Family Support Worker. Address discrimination effectively as a Family Support Worker. Develop essential qualities intrinsic to an effective Family Support Worker. Deliver crisis support with a Family Support Worker's skill set. Apply safeguarding laws in your role as a Family Support Worker. More Benefits: LIFETIME access Device Compatibility Free Workplace Management Toolkit Key Modules from Family Law, Family Support Worker and Safeguarding Diploma: Introduction to Family Law: Acquire Family Law basics essential for a Family Support Worker's role. Discrimination in Family Law: Identify and combat discrimination through a Family Support Worker's legal understanding. Qualities of a Family Support Worker: Cultivate the character and competencies necessary to excel as a Family Support Worker. Supporting Families in Crisis: Equip yourself with crisis management techniques tailored for a Family Support Worker. Laws and Guidance on Safeguarding: Integrate safeguarding laws and guidance into your practices as a Family Support Worker. Safeguarding of Vulnerable Adults: Ensure the well-being of vulnerable adults by applying principles aligned with a Family Support Worker's responsibilities.

CT14: Clinical Trial Safety Reporting Requirements in the EU and USA
By Zenosis
This course sets out the legal and regulatory requirements for safety reporting in clinical trials of medicinal products under the jurisdictions of the European Union and the USA. It builds on the foundation laid by our companion course CT13, Safety Reporting in Clinical Trials, and provides greater detail of specific requirements in those jurisdictions.

CT13: Safety Reporting in Clinical Trials
By Zenosis
This course explains the regulatory requirements for the reporting of adverse events and suspected adverse reactions in clinical trials. It describes how investigators should report to sponsors, and how sponsors should report to regulatory authorities and other stakeholders in the safety of investigational products. It explains how events are characterized as serious or non-serious, expected or unexpected, and it distinguishes the requirements for each category. It describes controlled vocabularies used for coding of events in reports.
CT11: How to Gain Authorisation for Clinical Research Under the EU Clinical Trials Regulation
By Zenosis
This course sets out the procedures that sponsors need to follow to gain authorisation to conduct clinical trials under the Regulation, and it summarises and links to the extensive guidance available from the European Commission and the European Medicines Agency. Its companion course CT12 sets out the procedures that sponsors need to follow to conduct authorised clinical trials in compliance with the Regulation. The two courses therefore provide an ideal foundation for understanding and complying with the new law.
Level 2 Youth Work Certificate & Social Worker Diploma - CPD Accredited
By Training Tale
Youth Work: Youth Work Certificate Course Online Introducing Youth Work: Youth Work Certificate Course "Empowering the Next Generation" Online course! Are you passionate about making a positive impact on young lives? Do you want to contribute to the development and growth of the next generation? If so, our online course Youth Work: Youth Work Certificate Course is tailored just for you! Our comprehensive Youth Work: Youth Work Certificate Course is designed to equip you with the knowledge, skills, and tools necessary to excel in the field of youth work. Whether you are a seasoned professional or someone looking to embark on a fulfilling career, this extensive Youth Work: Youth Work Certificate Course will provide you with valuable insights and practical strategies to effectively engage and empower young people. Join our Youth Work: Youth Work Certificate Course today and unlock your potential to make a lasting impact on young lives. Together, let's create a brighter future for the next generation! Enroll in our Youth Work: Youth Work Certificate Course now and be part of the change! Main Course: Level 2 Youth Work certificate Course Included Youth Work Certificate Course Along with Youth Work Certificate course, Learner will get free Level 5 Mental Health First Aid Certification Along with Youth Work Certificate course, Learner will get free Social Worker: Social Work Diploma Along with Youth Work Certificate course, Learner will get free Level 3 Designated Safeguarding Lead Training Along with Youth Work Certificate course, Learner will get free Level 3 Diploma for the Children and Young People's Workforce [Note: Free PDF certificate as soon as completing the Youth Work: Youth Work Certificate Course] Youth Work: Youth Work Certificate Course Online This Youth Work: Youth Work Certificate Course consists of 11 modules. Course Curriculum of Youth Work: Youth Work Certificate Course Module 01: Overview to Youth Work Module 02: Youth Workers: Who Are They? Module 03: Role and Approaches of Youth Worker Module 04: Ethical Conduct and Principles in Youth Work Module 05: Career as a Youth Worker (Social Work) Module 06: Social Influences on Youth Worker Module 07: The National Youth Agency (NYA) Module 08: The EU on Youth Work Module 09: Mental Health of Youth Worker Module 10: Dealing with Stress Module 11: Youth Justice and Social Work Assessment Method of Youth Work: Youth Work Certificate After completing each module of Youth Work: Youth Work Certificate course, you will find automated MCQ quizzes. To unlock the next module, you need to complete the quiz task and get at least 60% marks. Certification of Youth Work: Youth Work Certificate After completing the MCQ/Assignment assessment for this Youth Work: Youth Work Certificate course, you will be entitled to a Certificate of Completion from Training Tale which is completely free to download. Who is this course for? Youth Work: Youth Work Certificate Course Online This Youth Work: Youth Work Certificate Course is offered to students from all walks of life. Individuals working in similar fields can also register in this Youth Work: Youth Work Certificate Course to update or develop their abilities. Requirements Youth Work: Youth Work Certificate Course Online To enrol in this Youth Work: Youth Work Certificate Course, students must fulfil the following requirements: Youth Work: Youth Work Good command of the English language Youth Work: Youth Work Must be vivacious and self-driven Youth Work: Youth Work Basic computer knowledge Youth Work: Youth Work A minimum of 16 years of age is required Career path Youth Work: Youth Work Certificate Course Online Enrol in the Youth Work: Youth Work Certificate Course today to learn the skills and knowledge required to work with young people and organise youth activities. Certificates Certificate of completion Digital certificate - Included

Search By Location
- Healthcare Courses in London
- Healthcare Courses in Birmingham
- Healthcare Courses in Glasgow
- Healthcare Courses in Liverpool
- Healthcare Courses in Bristol
- Healthcare Courses in Manchester
- Healthcare Courses in Sheffield
- Healthcare Courses in Leeds
- Healthcare Courses in Edinburgh
- Healthcare Courses in Leicester
- Healthcare Courses in Coventry
- Healthcare Courses in Bradford
- Healthcare Courses in Cardiff
- Healthcare Courses in Belfast
- Healthcare Courses in Nottingham