- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
Executive Leadership
By Emergent Learning
Target Audience Senior managers with several years of experience and leaders looking to transition to the highest levels of leadership where the remit is characteristically more strategic, more authority, fewer direct reports and greater influence internally and externally. Duration 2 Days Course Overview The Executive Leadership course is an intensive program is for seasoned leaders who are responsible for large teams, departments, or entire organizations. It is designed to elevate senior leaders’ capabilities to excel in volatile, global, and digitally-driven contexts. Building on established leadership theories, this course dives deeper into advanced, research-backed models such as Complexity Leadership, Strategic Agility, and Ethical Governance, integrating insights from organizational psychology, behavioral economics, and neuroscience. Through high-level case studies, strategic simulations, and peer-to-peer dialogue, participants will refine their ability to lead transformative change, foster organisational resilience, and align purpose-driven strategies with stakeholder expectations. The course emphasizes practical application for navigating ambiguity, driving innovation, and ensuring sustainable impact. The Leadership Tension Lens will be revisited to help leaders more critically analyse competing strategic priorities and the most challenging leadership dynamics. This course is designed by highly qualified learning design experts, assisted and guided by a Doctoral & Masters level leadership team. Working closely with subject matter leaders with extensive domain experience, this course is built on sound academic rigour and applied real world experience. Run in a cohort-based, activity-led format, it goes beyond theory to provide practical methods and frameworks that you can immediately apply in your workplace. Key Outcomes Revisit the Leadership Tension Lens: Departmental Trade-offs and Tensions & Short Term v Long Term Define Organisation-wide Vision and Strategy - Strategic foresight Drive Organizational Performance: Metrics that Matter Apply Leadership at Scale: Building and Managing Executive Teams Explore Executive Coaching, Stakeholder Management Techniques Drive Cultural Transformation: Aligning Culture with Strategic Goals Apply Executive Reflection: Learning from Experience and Driving Change

Advanced Turnaround, Shutdown and Outage Management
By EnergyEdge - Training for a Sustainable Energy Future
Enhance your skills in turnaround, shutdown, and outage management with EnergyEdge's advanced classroom training. Enroll now!

The Auditing Course
By Research Quality Association
Course Information Designed to develop personal proficiency in audit planning, execution and reporting, this course is meticulously crafted to refine essential audit skill sets. Through immersive scenarios focused on on-site audit conduct (with an alternative Remote Auditing Course available), participants will engage deeply in the audit process. Extending Expertise: Applicable across all audit types, this course builds upon and enriches the foundational concepts taught in RQA's suite of research quality assurance courses. From 'Research Quality Assurance for Good Laboratory Practice' to 'Good Clinical Practice Auditing – Principles and Practice' and 'Good Manufacturing Practice for Investigational Medicinal Products,' this programme extends the scope of learning. Relevance and Value: Relevant to any area of regulated research and development, this course shines particularly in contexts mandating a quality system for audit. Participants with prior audit experience will gain maximum value from this course. Key Benefits: Enrich your skill set to: Navigate audit processes encompassing planning, execution, reporting, and follow-up Embrace a personalised approach fostering positive audit outcomes Analyse evidence and present cohesive audit findings Recognise the pivotal role of audits in driving continual improvement. Interactive Learning: Structured to foster dynamic engagement, this course encourages delegates to: Engage in discussions, idea development, and problem-solving Exchange invaluable information and experiences. Hands-On Experience: A highlight of this course is the series of practical workshops, where delegates work in small syndicate groups, applying the acquired skills from lectures into real-world scenarios. Tutors Tutors will be comprised of (click the photos for biographies): Andrew Waddell Founder Director, Tower Mains Ltd Rosemary Ichaba Senior QA Associate, Tower Mains Ltd Cate Ovington Director, The Knowlogy Group Ltd Jean McWilliam Associate Director, Alexion View pop up Programme Please note timings may be subject to alteration. Day 1 08:45 Registration 09:00 Welcome and Course Objectives 09:10 What is 'Audit'? Delegates explore the range of audits which they have experienced, define the purpose of each audit type and establish which of those audits are performed to meet regulatory requirements. 09:30 Audits and their Purpose The concepts of quality assurance, quality control, quality management and audit are discussed. 10:30 Break 10:45 Audit Planning The requirements for an effective audit programme and individual audit plans. 11:30 Workshop 1 - Getting the Audit Started Planning for the audit. 12:25 Workshop 1 - Feedback 12:45 Lunch 13:30 Workshop 2 - Getting the Audit Started Arranging the opening meeting. 13:50 Workshop 2 - Feedback Audit initiation. Review and discussion of the role of the opening meeting. 14:25 Auditing Techniques (1) - Data and Documentation Techniques for the conduct of data and report audits are investigated. 14:55 Break 15:10 Workshop 3 - Data and Documentation Audit Conducting an audit of a data package and supporting documentation. 17:15 Close of Day Day 2 09:00 Auditing Techniques (2) - The People Questioning techniques which get the required information from the auditee. 09:45 Live Audit Role Play Auditor and auditee behaviours are explored and strategies developed for successful audit interactions. 10:15 Break 10:35 Audit Closing Meeting An exploration of audit closing meetings. 11:00 Workshop 4 - Audit Observations and Preparing for the Closing Meeting Reviewing and categorising your observations and getting ready to present your case. 11:45 Workshop 4 - Feedback 12:30 Audit Reports The content and distribution of an effective audit report are investigated and the importance of effective written communication is discussed. 13:00 Lunch 13:45 Workshop 5 - Audit Reports and Follow-up Mechanisms for promoting effective corrective and preventive action. Critical review of an audit report example. 14:30 Workshop 5 - Feedback 14:55 Corrective and Preventive Action and Follow-up The auditor's role in monitoring responses to audit and the corrective and preventive actions promised is explored. 15:20 Panel Session An opportunity to get answers to outstanding questions. 15:30 Close of Course Extra Information Course material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. CPD Points 14 Points Development Level Develop

FAW & RFAW Training
By Certifi First Aid Training
For higher risk or larger workplaces/organisations such as engineering, factories, restaurants, warehousing, construction or for those who use chemicals. This is also the appropriate course for larger low risk workplaces or workplaces who have employees with medical conditions. No previous experience required HSE Compliant Certificate valid for 3 years Taught by experienced trainers Interactive & engaging delivery techniques All age groups covered (adults, children & infants) All learners will have the skills and knowledge to provide the organisation with First Aider’s that can provide treatment to their casualties in a prompt, safe and effective manner. Duration FAW Initial - 18 hours (2 days face to face + 6hrs of elearning) RFAW Requal - 12 hours (2 days face to face, with a valid previous certificate) Syllabus A range of subjects are covered including: Day 1 Role of the First Aider Primary Survey Unresponsive (Breathing) - Recovery Position & Monitoring Unresponsive (Not-Breathing) - CPR & AED Bleeding (Catesrophic, Severe & Minor) Burns Choking Seizures Shock Day 2 Fractures, dislocations and spinal injuries Poisoning Heart attacks Head injuries Chest injuries Asthma Stroke Shock Severe Allergic Reaction Eye injuries Sprains and strains Diabetes Day 3 (elearning) For those who are completing the qualification for the first time or do not have a valid existing certificate, will be required to complete elearning, which can be done before the training or within 30 days after the training. Those re-qualifying, need not complete the elearning. Assessment Continual assessment is used, combined with a number of observed practical activities and 3 short theoretical assessments, supported by our experienced trainers. Instructions Please ensure that this is shared with those who are attending the course Thank you for booking with Certifi Training, we are looking forward to having you on our course. You must bring a form of Photo ID to your training. This can be a passport, driving licence, government issued biometric card, rail card, Police Warrant Card or Official Work ID. Please arrive in good time for your training (we advise 15 mins before the start time). Late learners will not be accepted and will turned away without refund. Please wear clothing suitable for practical activities (no skirts or high heels). You must attend the entire course, learners who leave early will not be issued a certificate. Covid-19 - due to this being a healthcare course, you are prohibited from attending if you have contracted Covid-19. Practical Elements - in order to pass the course, you will be required to kneel down on the floor (primary survey), be rolled onto your side (recovery position) and perform 2 mins of rescue breaths and chest compressions (CPR). This will all have to be completed unaided. Cancellation and Postponement - our policy is found here (https://bookwhen.com/certifitraining/page/refund_policy) Injuries & Medical Conditions - if you have an injury or medical condition which would prevent you from performing practical activities, this may prevent us from being able to issue you with a qualification, please contact us if concerned. Allergies & Sensitivities - if you have an allergy or sesitivity towards hand santizers or cleaning products, please let your trainer know. Our equipment is latex free. Pregnancy - Please alert your trainer if you are or maybe pregnant. It will not stop you from being able to participate in the course, however your trainer will make a couple of adaptions during recovery position and choking activities. Reading & Writing - you must be able to read and write in English, however this can be supported by your trainer if you have a learning need (such as dyslexia) or English is your 2nd language. Certificates - certificates will be issued to the person who booked your course if you pass the course criteria. Certificates are in PDF format and will be issued within 2 weeks of the course. Elearning Certain Courses will be required to complete elearning, which can be done 30 days before or after the training, however it is beneficial if... [Read more]
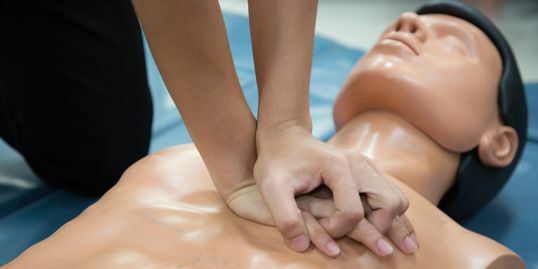
Good Clinical Practice Auditing - Principles and Practice
By Research Quality Association
Course Information Our comprehensive course is used as a gateway to those stepping into the world of auditing clinical studies. Tailored for those already acquainted with Good Clinical Practice (GCP) and those transitioning from other audit disciplines, this programme stands as a pivotal guide. Pre-existing knowledge of GCP will significantly enhance your learning experience in auditing against these guidelines. How is this course run? Engage in immersive workshops providing hands-on practice with auditing techniques in a GCP context. Our seasoned tutors, boasting extensive audit experience, intertwine theory with practical insights drawn from their own professional journeys. What will I learn? A comprehensive understanding of the historical backdrop and objectives driving Good Clinical Practice, incorporating the latest industry developments Solid grounding in quality assurance activities aligned with regulatory standards Insight into potential pitfalls within clinical trials and the pivotal role of auditors in addressing these issues Clarity on the roles and responsibilities inherent to clinical trials auditing Exposure to a diverse range of audit techniques complemented by illustrative examples and supportive documents A nuanced understanding of regulatory inspectors' activities Expanded professional networks to propel your auditing career forward. Benefits include: A clear understanding of the role of the auditor under Good Clinical Practice improved audits Improved Good Clinical Practice compliance for your clinical trials. This course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Examine particular aspects of Good Clinical Practice. Tutors Tutors will be comprised of (click the photos for biographies): Rosemarie Corrigan EVP Global Quality, Worldwide Clinical Trials Cathy Dove Director and Owner, Dove Quality Solutions Julie Kelly Associate Director, Clinical Quality Assurance, Corcept Therapeutics Susana Tavares Director of Research Quality Assurance, - Programme Please note timings may be subject to alteration. Day 1 12:30 Registration 13:00 Welcome and Objectives for the first day of the course 13:30 Laying the Foundations Introduction to the clinical development process, the concepts of quality assurance, quality control and audit. 14:30 Break 15:00 Patient Protection Requirements for informed consent and ethics committee. Access to source documentation. Including a patient protection exercise. 16:05 Workshop 1 - Case Study on Informed Consent 16:45 End of Day Questions and Answers 17:00 Close of Day Day 2 08:50 Questions and Answers from Day 1 09:00 Effective Site Audits The procedures involved in selecting and setting up audits at investigator sites. 09:40 Workshop 1 - Planning the Effective Audit 10:30 Break 10:45 Source Data Verification The need for and purpose of verifying data. 11:25 Workshop 2 - Source Data Verification 12:30 Lunch 13:30 IMP Management The requirements surrounding the distribution of investigational medicinal products. Accountability from release to destruction. 14:15 Critical Document Audits The conduct of other study specific audits including protocols, databases and reports. 15:00 Break 15:15 Non-compliance Determining the acceptability of data. 16:00 Fraud - Fact or Fiction? How to identify fraud and its consequences 16:45 End of Day Questions and Answers 17:00 Close of Day Day 3 08:50 Questions and Answers from Days 1 and 2 09:00 Auditing Third Parties A review of audits of contract research organisations. 10:00 System Audits The concept of auditing processes across many clinical trials, including a practical exercise in process mapping. 10:45 Break 11:00 Workshop 3 - Process Mapping 11:45 Effective Audits Where theory meets reality. 12:30 Lunch 13:20 Audit Reports - Closing the Loop An examination of the processes which follow the evidence gathering phase of the audit. 14:20 Workshop 4 - Audit Reports Audit reports, corrective and preventive action. 15:00 Break 15:10 Regulatory Inspection Auditors and regulatory inspections -how the QA team can help the organisation to perform during a regulatory inspection. 15:55 Final Questions and Answers 16:10 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 17 Points Development Level Develop

Motorcycle Wheel Building Masterclass (Online + 4 days of Private 1 to 1 tuition)
4.7(20)By Colwood Wheel Works
The Wheel Building Masterclass with Vince Warner Ready to Elevate Your Wheel Building Skills? Imagine Building Every Wheel with Confidence and Precision. That's What Mastery Feels Like. I started to learn to build wheels in 1967. My dad always said if you can count to 4 you can learn to build wheels, and as soon as I could count to 4 he had to prove to everybody that he was right. I was approaching my 4th birthday when he took me into his workshop and started to show me his trade secrets. I don't for one moment think you need to start at 3 years old to become an excellent wheel builder, but I do believe you need to be shown the techniques clearly by an experienced craftsman. Join Me in My Workshop for the Ultimate Learning Experience. What You'll Gain: Confidence in every wheel you build A high professional standard in all your projects The ability to charge for your expertise (if you wish to) The Masterclass Includes: Full access to all video training modules Personalised instruction from me, Vince Warner 4 full days of intensive training Exclusive private one-to-one attention A certificate showing you have completed the masterclass And Lunch is on Me, Every Day! Enjoy complimentary lunch, along with tea, coffee, cold drinks, and snacks throughout the day.

CPCS A77 Telescopic Handler Rotational
By BAM Construction Training Ltd
6 Days (Excluding Weekends) We run weekly open courses for accreditations including CPCS, CSCS Health & Safety, NPORS, IFAP, SMSTS and more. We also offer bespoke training courses or in house training nationwide. Upon arrival at our centres candidates will be inducted to the site by their qualified instructor who will be training them for the day. After registration candidates will be issued with a pack full of information relevant to the category they are being trained in. Included in this pack are theory questions, practical specifications, pre-start check lists, marking sheets and more. We ensure that these materials provided excel the individual to successfully complete their course. We are aware that having good quality machines give candidates a significant advantage when undertaking their training. We endeavour to supply candidates with the highest quality plant training and machinery on our site using a variety of certified suppliers. These machines come equipped with all the health and safety features and include – but are not limited to – JCB, Magni, Merlo, Caterpillar, Thwaits and Manitou. Get in touch with us today to find the right course for you. Our team are on-hand and ready to support you with your construction training requirements.

CPCS A59 Excavator 360 Above & Below 10T - Tracked
By BAM Construction Training Ltd
6 Days (Excluding Weekends) We run weekly open courses for accreditations including CPCS, CSCS Health & Safety, NPORS, IFAP, SMSTS and more. We also offer bespoke training courses or in house training nationwide. Upon arrival at our centres candidates will be inducted to the site by their qualified instructor who will be training them for the day. After registration candidates will be issued with a pack full of information relevant to the category they are being trained in. Included in this pack are theory questions, practical specifications, pre-start check lists, marking sheets and more. We ensure that these materials provided excel the individual to successfully complete their course. We are aware that having good quality machines give candidates a significant advantage when undertaking their training. We endeavour to supply candidates with the highest quality plant training and machinery on our site using a variety of certified suppliers. These machines come equipped with all the health and safety features and include – but are not limited to – JCB, Magni, Merlo, Caterpillar, Thwaits and Manitou. Get in touch with us today to find the right course for you. Our team are on-hand and ready to support you with your construction training requirements.

Diabetes Awareness
By Prima Cura Training
Diabetes is serious. It can be life-threatening, however, people with diabetes can live long, healthy lives if their condition is kept well-controlled. In this training course, we explain what diabetes is and what to look out for. We cover how it is diagnosed and how to provide care and support to a person living with diabetes.

Search By Location
- Driving Courses in London
- Driving Courses in Birmingham
- Driving Courses in Glasgow
- Driving Courses in Liverpool
- Driving Courses in Bristol
- Driving Courses in Manchester
- Driving Courses in Sheffield
- Driving Courses in Leeds
- Driving Courses in Edinburgh
- Driving Courses in Leicester
- Driving Courses in Coventry
- Driving Courses in Bradford
- Driving Courses in Cardiff
- Driving Courses in Belfast
- Driving Courses in Nottingham
