- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
The Complete Kotlin Developer Course
By Packt
Programming for Android and JavaScript made easy with the Kotlin language

Start your teaching career now. Self study, virtual and classroom courses. Are you ready to embark on an exciting journey into the world of teaching and training? Whether you aspire to teach in the Further Education & Skills Sector or deliver training in the workplace, our Level 3 Award in Education and Training (formerly known as PTLLS) is your gateway to success.

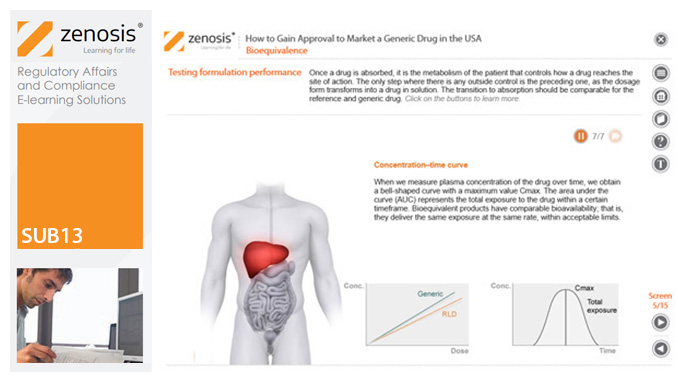
SUB13: How to Gain Approval to Market a Generic Drug in the USA
By Zenosis
This module outlines the legislative and regulatory context for the development of generic drugs and describes the essential role of the Abbreviated New Drug Application (ANDA) in gaining marketing approval. The use of information in the ‘Orange Book’ is explained, as is the role of patent certification in the application. The importance of establishing bioequivalence between a generic and its reference product is emphasised. The module specifies the content and format requirements for an ANDA submission and describes the FDA’s review and approval process. An outline is given of the Generic Drug User Fee Amendments (GDUFA) and the law’s effects on industry players.
Program Management Professional (PgMP) Exam Prep Course
By Packt
This course will take you through the concepts of PMBOK (Project Management Body of Knowledge) and will prepare you for the PgMP certification exam. With the help of this course, you will be able to access the lesson-end quizzes and course-end assessments, which will prepare you for the certification exam.






