- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
485 Courses delivered Online
Mastering Persuasive Communication for Sales
By Compete High
ð Unlock the Power of Persuasion and Elevate Your Sales Game! ð ð Are you ready to revolutionize your sales approach and achieve unparalleled success? Welcome to 'Mastering Persuasive Communication for Sales' - your gateway to becoming a persuasive sales powerhouse! ð ð¥ Imagine having the ability to captivate your audience effortlessly, compelling them to say 'Yes!' at every turn. This comprehensive online course is meticulously crafted to equip you with the indispensable skills needed to become a persuasive maestro in the realm of sales. ð¯ Here's what awaits you on this transformational journey: ð¡ Proven Strategies: Gain exclusive access to battle-tested techniques and strategies employed by top sales professionals worldwide. Learn how to craft compelling narratives, leverage psychological triggers, and master the art of persuasion to influence decisions effectively. ð Communication Mastery: Discover the keys to unlocking the secrets of effective communication. From honing your body language to perfecting your tone and words, you'll learn how to create an irresistible magnetism in every interaction. ð Sales Psychology Unveiled: Delve into the psychology behind customer behavior and decision-making processes. Understand the core principles that drive buying decisions, enabling you to anticipate needs and guide prospects seamlessly through the sales funnel. ð Online & Offline Mastery: Whether you're engaging face-to-face or navigating the digital landscape, this course covers both online and offline persuasive communication techniques, ensuring you're equipped for any sales scenario. ð Real-World Applications: Put theory into action with practical exercises, role-playing scenarios, and case studies that simulate real-world sales situations. Sharpen your skills in a risk-free environment and gain the confidence to excel in any sales conversation. ð Expert Guidance: Led by industry experts with a wealth of experience in sales and communication, this course provides unparalleled guidance, offering insights and tips that can only be gained from years of successful practice. ð Elevate your sales game and skyrocket your success! Enroll now in 'Mastering Persuasive Communication for Sales' and unlock the doors to a whole new level of sales prowess. Your journey towards becoming a persuasive communication virtuoso begins today! ðð¼ Course Curriculum INTRODUCTION Introduction 00:00 My Story 00:00 SALES SCRIPTING TACTICS AND STRATEGIES Embracing Sales 00:00 Embrace Sales Scripting 00:00 The 7 Step Script Writing Formula 00:00 The 5 Laundry Lists - Part 1 00:00 The 5 laundry lists - Part 2 00:00 Sales Scripting Techniques - Part 1 00:00 Sales Scripting Techniques - Part 2 00:00 CONCLUSION Scripting Best Practices and Conclusion 00:00

Learning & Development Level 5
By Rachel Hood
Ensuring learning and development contributes to improved performance in the workplace at an individual, team and organisation level.

Process Mapping and Standard Operating Procedure (SOP) Writing
By Research Quality Association
Course Information This course aims to empower you with the expertise to proficiently navigate process mapping and master the art of crafting Standard Operating Procedures (SOPs) within regulated environments. Whether you're engaged in activities that demand process improvement, continual enhancement, or SOP creation, this course offers invaluable insights tailored to your needs. It caters to individuals tasked with managing, documenting, and implementing processes and SOPs, irrespective of prior experience or skills. Our curriculum does not focus on specific software or approaches, focusing instead on fundamental principles and adaptable concepts applicable across diverse organisational landscapes. Benefits include: Hands-on experience in mapping processes An understanding of how process maps can be used to define, communicate and continually improve complex processes An understanding of best practice for SOPs using of process maps/process flow diagrams and process mind maps to complement text An insight into the preparation of concise and user friendly SOPs. This course is structured to encourage delegates to: Discuss and develop ideas Develop a practical approach for creating process maps and writing SOPs Understand how to use process maps in SOPs effectively Discuss how process mapping for preparing SOPs can be applied to process improvement for SOPs. Is this course for you? The course is designed for all those with responsibility for managing, documenting and implementing processes and SOPs. It assumes no prior experience or skills. The course does not recommend any specific software or approach, but explores the principles and ideas that can be applied in any organisation. What will you learn? Hands-on experience in mapping processes An understanding of how process maps can be used to define, communicate and continually improve complex processes An understanding of best practice for SOPs using of process maps/process flow diagrams and process mind maps to complement text An insight into the preparation of concise and user friendly SOPs. By the end of the course you will be able to: Create process maps and write clearer more concise SOPs Understand how to use process maps in SOPs effectively Understand how process mapping can be applied to process improvement and better SOPs. Tutors Tutors will be comprised of (click the photos for biographies): Laura Brown Director, Laura Brown Training and Development David Butler VP of Quality, Resolian Programme Please note timings may be subject to alteration. Day 1 09:00 Welcome, Introductions and Course Objectives Why Companies manage by process. 10:00 Exercise 1 - First Steps Mechanisms for process mapping, levels at which it can be applied, and the roles and responsibilities of process owners. 10:30 Exercise 1 - Feedback 10:45 Break 11:00 Mapping Processes The stages involved in mapping processes including how to construct a SIPOC chart. 11:45 Exercise 2 - Mapping A Process A first opportunity to practice your new process mapping skills. 12:30 Exercise 2 - Feedback 13:00 Lunch 14:00 Exercise 3 - Discussion of Options for Format, Structure and Layout What Makes A Good SOP? Discussion of options for format, structure, level of detail of SOPs and the use of process maps for SOP writing. A case study example of a good SOP which uses a flow chart/process map. 14:45 Exercise 3 - Feedback 15:00 Break 15:15 A Case Study example of a good SOP that uses a flow chart / process map 15:30 Exercise 4 Discussion of the content of an SOP using a Process Map 15:35 Exercise 4 - Feedback 15:45 Key Writing Considerations 16:15 Exercise 5 16:45 Exercise 5 - Feedback 17:00 Close of Day Day 2 09:00 Review of Day 1 09:15 Using Metrics Selecting and using metrics to monitor and improve processes. 10:00 Exercise 6 - Practice in Process Mapping Creating Process Maps from multi-source information. 11:00 Break 11:15 Exercise 6 - Feedback 11:45 Common Pitfalls Common problems and tips for good Process Mapping. 12:15 Demonstration of Process Mapping on a PC 12:45 Lunch 13:30 Exercise 7 - Mind Mapping 14:30 Exercise 7 - Feedback 15:00 Break 15:15 Exercise 8 - Using Process Maps to Write an SOP Applying your process mapping skills as part of writing an SOP 15:30 Exercise 8 - Feedback 15:45 Course Review and Follow-up 16:00 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. Development Level Learn

The Magic of Mentoring
By Mpi Learning - Professional Learning And Development Provider
In this course you will understand what mentoring is about, understand your roles in the process. Gain self-insight into your own interpersonal style and way of relating to others that may affect mentoring interactions.

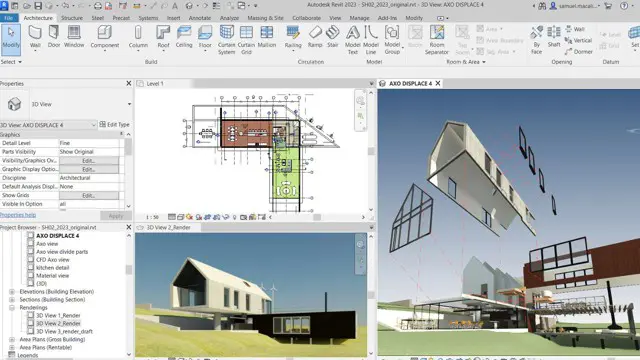
Revit One to One Basic to Advance Weekends Online or Face to Face
By Real Animation Works
Revit face to face training customised and bespoke. Online or Face to Face
Teaching Online, Business English, Young Learners and Test Preparation Courses (25 Hours)
5.0(5)By TEFL Wonderland - Brilliant Minds
Teaching Online, Business English, Young Learners and Test Preparation Courses (25 Hours)

Level 3 Award in Education and Training (RQF) & Aviation Train the Trainer
5.0(37)By ATC Training Limited
The Level 3 Award in Education and Training (RQF) formerly known as PTLLS, has been designed to introduce teaching for individuals who aspire or currently work in adult education. This includes FE colleges, training providers, workplace training departments and/or Local Authorities. It is a nationally recognised qualification and is also often the preference for employers to source as an accredited train the trainer qualification. It is recognised by the UK Civil Aviation Authority (CAA) as an acceptable qualification to become a CAA certified trainer. This version of the course has been further developed to meet the requirements of the IATA Train the Trainer syllabus with additional aviation related content, our course is aligned with IATA AHM1110 11.2 (GEN10). This course can also be used to meet the standards of IS-BAH IG 8.1.6. This qualification includes three written assignments and a practical micro-teach session. Free online live sessions are also available.

Good Clinical Practice Auditing - Principles and Practice
By Research Quality Association
Course Information Our comprehensive course is used as a gateway to those stepping into the world of auditing clinical studies. Tailored for those already acquainted with Good Clinical Practice (GCP) and those transitioning from other audit disciplines, this programme stands as a pivotal guide. Pre-existing knowledge of GCP will significantly enhance your learning experience in auditing against these guidelines. How is this course run? Engage in immersive workshops providing hands-on practice with auditing techniques in a GCP context. Our seasoned tutors, boasting extensive audit experience, intertwine theory with practical insights drawn from their own professional journeys. What will I learn? A comprehensive understanding of the historical backdrop and objectives driving Good Clinical Practice, incorporating the latest industry developments Solid grounding in quality assurance activities aligned with regulatory standards Insight into potential pitfalls within clinical trials and the pivotal role of auditors in addressing these issues Clarity on the roles and responsibilities inherent to clinical trials auditing Exposure to a diverse range of audit techniques complemented by illustrative examples and supportive documents A nuanced understanding of regulatory inspectors' activities Expanded professional networks to propel your auditing career forward. Benefits include: A clear understanding of the role of the auditor under Good Clinical Practice improved audits Improved Good Clinical Practice compliance for your clinical trials. This course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Examine particular aspects of Good Clinical Practice. Tutors Tutors will be comprised of (click the photos for biographies): Rosemarie Corrigan EVP Global Quality, Worldwide Clinical Trials Cathy Dove Director and Owner, Dove Quality Solutions Julie Kelly Associate Director, Clinical Quality Assurance, Corcept Therapeutics Susana Tavares Director of Research Quality Assurance, - Programme Please note timings may be subject to alteration. Day 1 12:30 Registration 13:00 Welcome and Objectives for the first day of the course 13:30 Laying the Foundations Introduction to the clinical development process, the concepts of quality assurance, quality control and audit. 14:30 Break 15:00 Patient Protection Requirements for informed consent and ethics committee. Access to source documentation. Including a patient protection exercise. 16:05 Workshop 1 - Case Study on Informed Consent 16:45 End of Day Questions and Answers 17:00 Close of Day Day 2 08:50 Questions and Answers from Day 1 09:00 Effective Site Audits The procedures involved in selecting and setting up audits at investigator sites. 09:40 Workshop 1 - Planning the Effective Audit 10:30 Break 10:45 Source Data Verification The need for and purpose of verifying data. 11:25 Workshop 2 - Source Data Verification 12:30 Lunch 13:30 IMP Management The requirements surrounding the distribution of investigational medicinal products. Accountability from release to destruction. 14:15 Critical Document Audits The conduct of other study specific audits including protocols, databases and reports. 15:00 Break 15:15 Non-compliance Determining the acceptability of data. 16:00 Fraud - Fact or Fiction? How to identify fraud and its consequences 16:45 End of Day Questions and Answers 17:00 Close of Day Day 3 08:50 Questions and Answers from Days 1 and 2 09:00 Auditing Third Parties A review of audits of contract research organisations. 10:00 System Audits The concept of auditing processes across many clinical trials, including a practical exercise in process mapping. 10:45 Break 11:00 Workshop 3 - Process Mapping 11:45 Effective Audits Where theory meets reality. 12:30 Lunch 13:20 Audit Reports - Closing the Loop An examination of the processes which follow the evidence gathering phase of the audit. 14:20 Workshop 4 - Audit Reports Audit reports, corrective and preventive action. 15:00 Break 15:10 Regulatory Inspection Auditors and regulatory inspections -how the QA team can help the organisation to perform during a regulatory inspection. 15:55 Final Questions and Answers 16:10 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 17 Points Development Level Develop

Louise Tremayne - Stress and Anxiety Specialist
By Free Flowing Energy
I am an experienced EFT Practitioner, Mindfulness Teacher, Therapist and Coach who specialises in helping women eliminate stress and anxiety. I offer sessions worldwide via the internet, or face to face in Halwill Junction, Okehampton and Exeter, Devon, UK. I also teach classes and run retreats. I love to empower my clients so that they can help themselves. I would love to hear from you, so please do get in touch.

