- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
7049 Courses in Elland delivered Live Online
LOOKING FOR: YA, NA, ADULT FICTION Helen Lane has been an established agent for several years, formerly with the Booker Albert Agency before joining the Ki Agency. She has a BSc in Environmental Science from the University of East Anglia and a postgrad qualification in Acoustics. She lives in London with her family and the world’s naughtiest cocker spaniel. Helen represents (Adult) Fantasy, Sci Fi, Horror, Romance, Action & Adventure, and Thrillers. She also represents select YA Fantasy, Horror, and Sci Fi. Her tastes do run towards the dark and she is slightly obsessed with monster stories in general (especially if they have giant sharks or squids). And stories set in extreme environments such as the arctic, jungle, caves, ocean, and mountains. But she DOES NOT want to read about affairs or abuse in any genre. (Her husband likes her to add here that this is nothing to do with him, she just doesn’t like sad stories). Her favourite authors are Mira Grant, Greig Beck, James Rollins, Matthew Reilly, Darcy Coates, Andy Weir, Clive Cussler, R F Kuang, Patricia Briggs, and Kelley Armstrong. Her comfort movies are: Jaws, Outbreak, The Cave, Sanctum, Scream, Buffy, Grey's Anatomy, Charmed, Vampire Diaries. You can find her on: Bluesky: @helenlane.bsky.social Instagram: hflane_agenting Helen would like you to submit a covering letter, 1 page synopsis and first three chapters (Max 5,000 words) of your manuscript in a single word document. (In addition to the paid sessions, Helen is kindly offering one free session for low income/under-represented writers. Please email agent121@iaminprint.co.uk to apply, outlining your case for this option which is offered at the discretion of I Am In Print). By booking you understand you need to conduct an internet connection test with I Am In Print prior to the event. You also agree to email your material in one document to reach I Am In Print by the stated submission deadline and note that I Am In Print take no responsibility for the advice received during your agent meeting. The submission deadline is: Wednesday 3rd September 2025

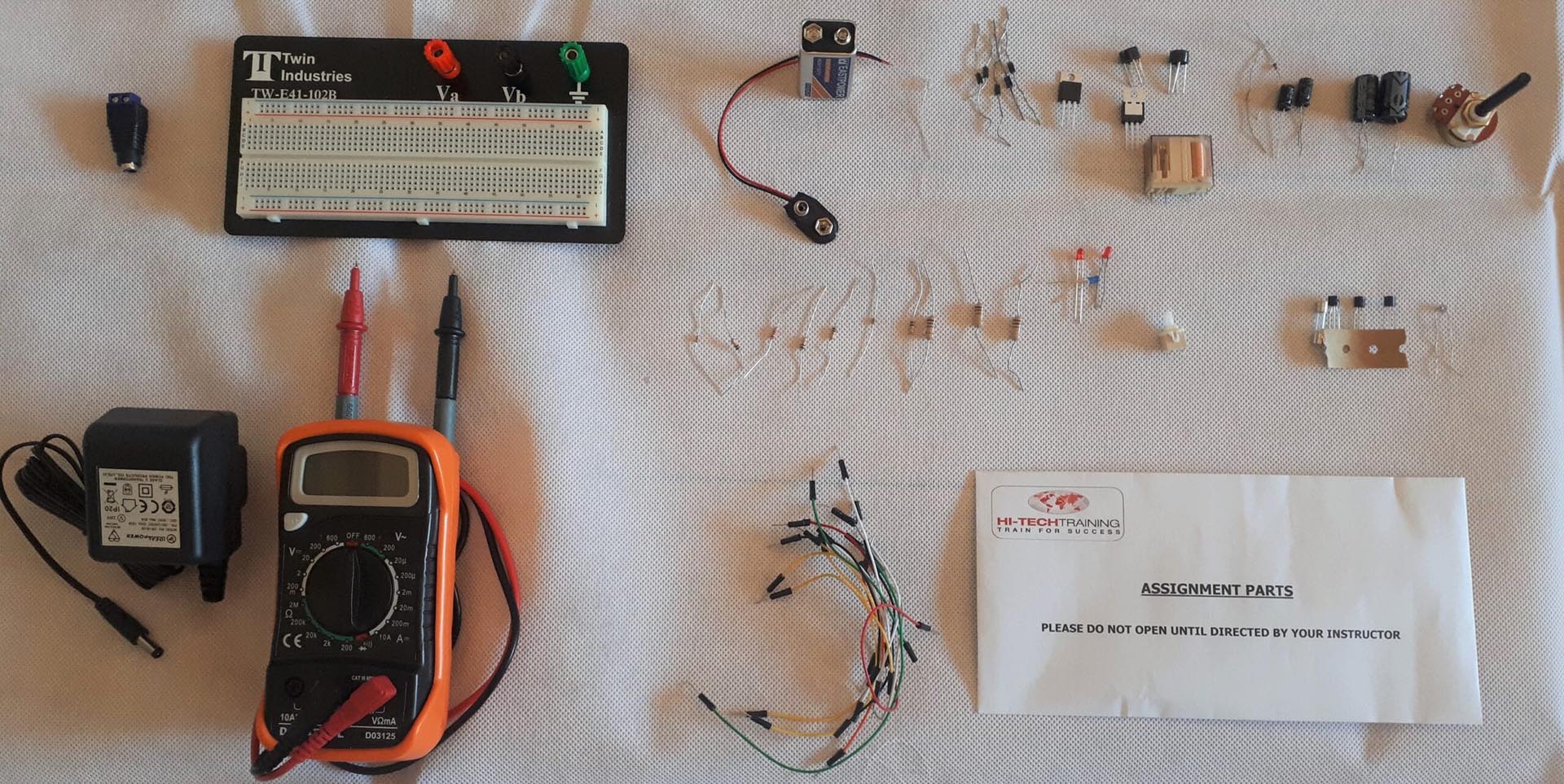
Electronics Repair 1 Course
By Hi-Tech Training
The Electronics Equipment Repair 1 equips participants with practical “Hands-On” skills relevant to the workplace and the theory required for certification. Participants on successful completion of the course will have the skills and knowledge to: Demonstrate the operation of a wide range of electronic components and circuits and their applications in modern electronic-based equipment such as amplifiers, Hi-Fi systems, stereos, and control systems Construct, test and fault-find the following popular basic circuits: Power supplies, amplifiers, timers, etc. Become competent in the correct use of electronic test and measurement equipment such as Analogue and Digital Multimeter and Oscilloscopes.
City & Guilds Electric Vehicle Charging Equipment Installation
4.7(1243)By Technique Learning Solutions
This qualification aims to provide expert guidance to learners wishing to gain knowledge and understanding on Electric Vehicle charging equipment installation. This 2 day course provides expert guidance on EV charging equipment installation, an important emerging area which is not covered in detail by the current edition of the Wiring Regulations (BS 7671) or the IET’s Guidance Notes. Aimed at experienced electricians interested in understanding a wide range of equipment and systems available, this course applies to the specialised installation requirements of electric vehicle charging equipment in domestic dwellings, on-street locations, commercial and industrial premises. The course provides detailed guidance and recommendations on all aspects of the installation of Electric Vehicle Charging Equipment from the origin of the electrical supply, through distribution and final circuits, installation of the charging equipment itself to the cable between the charging equipment and vehicle’s electrical inlet. Also included are related issues of site layout and planning and subsequent inspection, testing, certification and maintenance of installations. Why take this course? Currently there is an increasing demand for new electric vehicle charging points but too few installers to roll them out. This is already a booming market which is due to get much bigger in the near future. The number of public charging connectors and locations has increased by 38% in the past 12 months and is expected to continue at that rate for years to come. Entry Requirements There are no formal entry requirements for this qualification, however we do expect you to meet the following requirements: Minimum age 18 years old (mandatory) Must be able to demonstrate the following competencies Be able to correctly install and terminate: pvc/pvc cable (twin and earth) Steel Wire armoured cable (swa) Be able to carry out an initial verification (inspection & testing) on an electrical installation and complete the necessary paperwork. Please Note: These competencies are required for the assessment and are not taught as part of the course. It is also recommended that you are up to date with your wiring regulations.

Tackle Stress Before It Tackles You! Work-related stress affects 875,000 people every year, and its impacts go beyond the workplace—affecting your mind, body, and personal life. But it doesn’t have to be this way. Join our Stress Management Workshops to: ✔️ Understand the difference between stress and pressure ✔️ Learn the causes of stress in and out of the workplace ✔️ Discover practical coping strategies and build mental resilience These workshops are packed with insights, tools, and strategies to help you take control of your stress levels and improve your well-being—personally and professionally. There are two different ones to choose from - a 2 hour workshop and a 4 hour workshop! Course Contents of 2 hour course: What is Stress Stress versus Pressure Statistics Absenteeism, Presenteeism and Leaveism Workplace Causes of Stress Personal Causes of Stress Short-Term and Long-Term Effects of Stress Coping Strategies Mental Resilience Benefits of this Workshop: In 2022/23. 875,000 people suffered from work-related stress, depression or anxiety The affects of stress are far reaching, affecting one's mind, body, social and personal life Become more aware of what stresses you, what is does to you and find ways to reduce those stress levels

Alarm Installation Course
By Hi-Tech Training
The Alarm Installation Course is designed to teach participants how to install an intruder alarm system in domestic, commercial or industrial premises. The Alarm Installation Course simulates the practical installation of many different alarm control panels. The course is designed to equip students with the skills and expertise to competently install a wide variety of Alarm systems on the market.

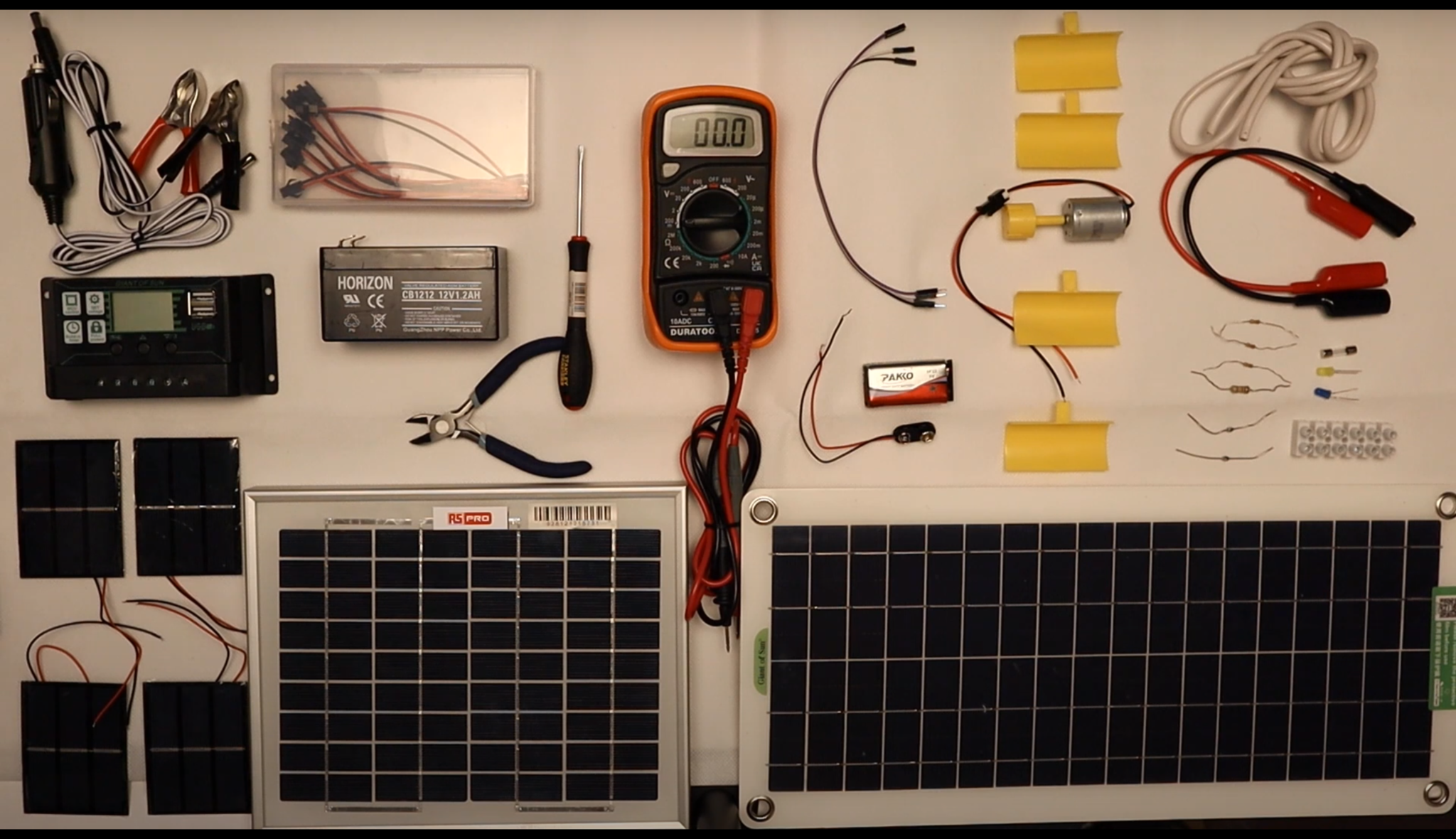
Alternative Energy Technology Course
By Hi-Tech Training
The Alternative Energy Technology Course is a practically based alternative energy course designed to give participants an understanding of alternative energy options and provide them with that practical knowledge and skills to build solar to electric and wind to electric systems at a foundation level. The course is designed to be of benefit to people either working or intending to work as:- Alternative Energy Installers or anyone just wishing to gain practical knowledge of Practical Alternative Energy Systems
11th September Emily MacDonald #Agent121. Looking for: ADULT FICTION, NON-FICTION
5.0(3)By I Am In Print
LOOKING FOR: ADULT FICTION, NON-FICTION Emily MacDonald joined 42 management and production company in 2019, working with Eugenie Furniss across her clients. She is now an agent in the Books Department, and is building her own list. Send Emily a story with characters that just won't leave her and a narrative that pulls her in, keeping her immersed in the world well after she has finished reading. She reads across a wide-range and has a particular interest in: book club, high-concept crime/thriller, upmarket and literary fiction. In fiction, Emily would love to find a crime/thriller set within a subcult: a modern retelling of British folklore (think Kamila Shamsie’s Home Fire); an unconventional love story; and a character led journey of self-discovery (especially one set within a diasporic community). Emily is also looking for narrative non-fiction which immerses the reader into an untold true story (personal or historical), exploring a new point of view, and providing a compelling social commentary, with an investigative twist. Think the obsessive vein of Kirk W. Johnson or personal/political dispatches of Aidan Hartley. Emily wants her horizons to expand while she reads. In both fiction and non-fiction, Emily loves stories woven into their surroundings, where the setting is as central a character as those who drive the narrative. In both spheres, she is keen to hear from Scottish and regional voices with stories to tell. Please note, Emily is not currently accepting submissions for Children's lit/YA and SFF. Emily would like you to submit a covering letter, 1 - 2 page synopsis and the first 5,000 words of your manuscript in a single word document. (In addition to the paid sessions, Emily is kindly offering one free session for low income/under-represented writers. Please email agent121@iaminprint.co.uk to apply, outlining your case for this option which is offered at the discretion of I Am In Print). By booking you understand you need to conduct an internet connection test with I Am In Print prior to the event. You also agree to email your material in one document to reach I Am In Print by the stated submission deadline and note that I Am In Print take no responsibility for the advice received during your agent meeting. The submission deadline is: Thursday 4th September 2025

Existential Dialogues 2025: “In-Sanity, Who is Sane?” with Prof E. Spinelli
By Therapy Harley Street
Ten live dialogues between Prof Ernesto Spinelli and International Existential Therapists followed by an Experiential Study Group. A phenomenological approach to re-view psychopathology: We aim to explore the lived experiences of irregular perceptions of reality with an open mind. Each Saturday includes a live dialogue between Prof Ernesto Spinelli and an International Existential Therapist; a moment to share your thoughts and feelings with the teachers; and a final integration facilitated by Bárbara Godoy. This series of ten Dialogues set out to explore the multifaceted dimentions and complexities associated with Existential Therapies. It attempts to engage with various interpretations of insanity through the lens of patients' often painful, confounding, and deeply unsettling life experiences. TIMES AND DATES 2025 25 Jan. “Knots” with Prof. Ernesto Spinelli 2 Feb. “Healing” with Dr. Michael Guy Thompson 22 Mar. “Difference” with Dr. Todd DuBose 12 Apr. “Polarization” with Prof. Kirk Schneider 3 May “Character” with Prof. Robert Romanyshyn 21 Jun. “Opening” with Dr. Yaqui Martinez 19 Jul. “Meaning” with Dr. Jan Resnick 25 Oct. “Invention” with Dr. Betty Cannon 15 Nov. “Hallucination” with Prof. Simon du Plock 13 Dec. “Hysteria” with Bárbara Godoy Full course (including dialogues): £1260 (2 pm to 5 pm – UK time) Only Dialogues: £630 (2 pm to 3 pm – UK time) Venue: Online Zoom Read the full Programme here > Course Organised by:

Thriving at Work: A Neurodivergent Perspective
By Emergent Learning
Target Audience This course is for people who are either neurodivergent or working with neurodivergent colleagues. It will help you work more effectively by understanding the needs and recognising the strengths of neurodivergence. This will help you develop strategies to support performance and enhance wellbeing in the workplace. Duration 1 Day Course Overview This course is designed for teams with neurodivergent professionals who want to better navigate work environments, build on their strengths, and manage friction in everyday tasks. Through practical tools, reflection, and real-life strategies, you'll gain greater clarity and confidence in how you work best — and how to advocate for what you need to thrive. Participants will explore a range of neurodivergent profiles and learn how to adapt communication, adjust workflows, reduce workplace triggers, and advocate for a fairer and more inclusive culture. This course is designed by highly qualified learning design experts, assisted and guided by a Doctoral & Masters level leadership team. Working closely with subject matter leaders with extensive domain experience, this course is built on sound scientific & academic rigour and applied real world experience. Run in a cohort-based, activity-led format, it goes beyond theory to provide practical methods and frameworks that you can immediately apply in your workplace. Course Objectives Recognise how neurodivergent traits shape focus, energy, and work patterns Develop strategies and adjustments that support strengths and reduce friction Communicate needs and preferences to improve collaboration and confidence High Level Topics Neurodiversity and Workplace Impact Working with Strengths and Challenges Strategies for Everyday Success

