- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
Fast Track To Aesthetics - Dermal filler training
By Cosmetic College
Are you looking to start a career in aesthetics but need the prerequisites for a traditional course? Look no further! Cosmetic College offers a fast-track pathway to becoming an accredited and insurable cosmetic injector. Our five day course is designed specifically for non-medics who want to pursue a career in aesthetics but need to meet the standard course prerequisites. Expert practitioners and educators deliver our course with years of industry experience. We prioritise safety and ensure that all learners will leave the system with the competency and knowledge necessary to perform injectables and aesthetic procedures with confidence. Before starting the course, you'll complete online pre-study and virtual lectures to give you a solid foundation in anatomy and physiology, health and safety, contraindications, complications, first aid, and infection control. Then, you'll spend 6-8 days of practical training, depending on your chosen course path, to hone your skills and get hands-on experience. Our fast-track pathway to injectables covers various topics, including microneedling, B12 dermaplaning, foundation lip dermal fillers, and more. To enrol in our fast-track course, you must be at least 21 years of age and have a good command of English. You should also be able to learn independently and have a strong desire to build a career in aesthetics. Invest in your future with Cosmetic College's fast-track pathway to injectables. With our comprehensive curriculum, expert instructors, and hands-on training, you'll be ready to start your aesthetic career. Take advantage of this opportunity to fast-track your aesthetic career. Enrol in our fast-track pathway to injectables today! Course Entry Requirements: Minimum age of 21 years Good command of English Be able to learn independently A strong desire to build a career in Aesthetics Course Pre-Study/Practical & Length: Online study and virtual lectures A series of online assessments Five days of practical training Course Agenda: Microneedling B12 Dermaplaning Foundation Lip Dermal Fillers Anatomy and Physiology Anaphylaxis Awareness Infection control Sharps and hazardous waste Complication management First aid Course Benefits Student Benefits Comprehensive Education: Our training program provides a comprehensive education on dermal fillers, equipping you with the knowledge and skills necessary to excel in the field of aesthetics. You will gain a deep understanding of facial anatomy, product selection, injection techniques, safety protocols, and patient assessment. Practical Hands-on Experience: We emphasise practical training, giving you ample opportunities to practice your skills under the guidance of experienced trainers. Working with live models will enhance your confidence and proficiency in performing dermal filler treatments. Industry-Standard Techniques: You will learn industry-standard techniques for administering dermal fillers, ensuring that you provide safe and effective treatments to your clients. This knowledge will enable you to achieve natural-looking results and meet the expectations of your clients. Personalised Guidance and Mentorship: Our trainers are highly experienced professionals who will provide personalised guidance and mentorship throughout the training. They will address your questions, offer constructive feedback, and help you refine your techniques, ensuring your continuous growth and improvement. Business and Marketing Support: In addition to the technical skills, we provide guidance on the business aspects of aesthetics. You will learn strategies for building and marketing your aesthetics practice, attracting clients, and maximizing your earning potential. This knowledge will empower you to establish a successful career in aesthetics. Client Benefits Expertise and Safety: Clients will have confidence knowing that you have received comprehensive training in dermal fillers. Your expertise will enable you to provide safe and high-quality treatments, minimising the risks and complications associated with the procedures. Natural and Desired Results: Through your training, you will be able to deliver natural and desired results to your clients. Your understanding of facial anatomy, product selection, and injection techniques will allow you to enhance their features, rejuvenate their appearance, and boost their self-confidence. Professionalism and Trust: By enrolling in our training program, you demonstrate your commitment to professionalism and continuous learning. Clients will trust in your skills and knowledge, knowing that you have received proper training from a reputable institution. Earning Potential Earning potential in the aesthetics industry can be significant. As a trained professional in dermal fillers, you can offer sought-after services to clients. The increasing demand for aesthetic treatments, combined with your expertise and ability to deliver exceptional results, can lead to a thriving client base and increased earning potential. The Fast Track To Aesthetics - Dermal Filler Training provides you with the foundation and skills necessary to capitalise on these opportunities and maximise your earning potential in the aesthetics field. Frequently Asked Questions Is this training suitable for beginners with no prior experience in aesthetics? Absolutely! Our Fast Track To Aesthetics program is designed to cater to beginners who are new to the field of aesthetics. We provide comprehensive training that covers the fundamentals of dermal fillers, ensuring that you gain the necessary knowledge and skills to start your journey in aesthetics. What topics are covered in the training program? Our training program covers a wide range of topics including facial anatomy, patient assessment, product selection, injection techniques, safety protocols, and client management. You will also receive hands-on training with live models to practice your skills under the guidance of experienced trainers. Are the trainers experienced in the field of aesthetics? Yes, our trainers are highly experienced professionals with extensive knowledge and practical experience in the field of aesthetics. They are dedicated to providing you with the guidance and support you need to excel in your training.

Peripheral Intravenous Cannulation (Peripheral vascular access device)
By Guardian Angels Training
Gain the knowledge and skills to safely insert and manage peripheral IV cannulas with our training course for healthcare professionals. Ideal for nurses, medical personnel, and other practitioners.
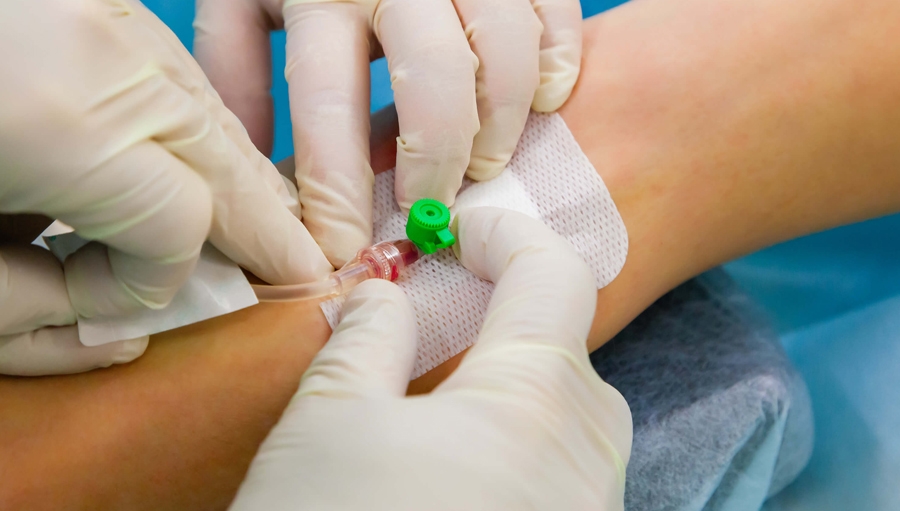
Intravenous Administration of Fluid and Medication
By Guardian Angels Training
Gain the knowledge and skills for safe and effective intravenous therapy with our "Intravenous Administration of Fluid and Medication" course. Ideal for healthcare professionals administering IV fluids and medications.
Level 3 Endorsed Award in Delivering Health and Social Care Training (Healthcare Train the Trainer)
By Guardian Angels Training
Gain expertise in healthcare training with our Level 3 Endorsed Award in Delivering Health and Social Care Training. Our comprehensive program equips you with the skills and knowledge to become a proficient trainer in the healthcare sector.

Foundations of Immunisation and Vaccines for Non-Registered Practitioners
By Guardian Angels Training
The "Foundations of Immunisation and Vaccines for Non-Registered Practitioners" course is fully compliant with the National Minimum Standards and Core Curriculum for Immunisation Training and is designed to equip non-registered healthcare professionals with a solid understanding of immunisation concepts, vaccine administration, and the importance of vaccination in public health.
This 1x day course is designed to teach you an abundance of taping techniques whilst providing you with the skills and understanding to effectively apply kinesiology tape to any MSK condition The course is aimed at Manual therapists including; Physiotherapists, Osteopaths, Chiropractors, Sports therapists, Massage Therapists and Personal Trainers. Course Summary This is a one day course consists of both theory and practical elements to provide you with the knowledge and skills required to feel confident and competent in using kinesiology tape with your clients. The day is designed to be an informal continued professional development opportunity with no formal assessment process. With our expert tutors on hand we will provide continued support throughout the day to ensure you gain and achieve everything you want from the course. Venue BTST Academy & Clinic, Holly Farm, Clipstone Road, Edwinstowe, Nottingham, NG21 9JD Course Times Start 9:30am – Finish 4:30pm Course Price £175 Tutor Laura Simmons Course Summary This is a one day course consists of both theory and practical elements to provide you with the knowledge and skills required to feel confident and competent in using kinesiology tape with your clients, understanding how to help prevent & manage injuries. The day is designed to be an informal continued professional development opportunity with no formal assessment process. With our expert tutors on hand we will provide continued support throughout the day to ensure you gain and achieve everything you want from the course. Course Content – Introduction -What is Kinesio Tape -History of Taping -Taping to treat… -Theory of Taping -Pain Gate Theory -About Tape -Contractions & Contraindications -Golden Rules -Tape Application Conditions Covered Plantar-Fasciitis, Achilles Tendinopathy, Muscular Tightnesses & Strains, Medial & Lateral collateral ligament strain, Lower Back pain / pathologies, CDJ Dysfunction, Tennis & Golfers Elbow, Rotator Cuff impingement, Postural taping and more! Course has been designed for; Physiotherapists Osteopaths Chiropractors Manual Therapists Sports Therapists Sports Massage Therapists Personal trainers Assessment Observation during the course day Course Terms & Conditions & Cancellation Policy Click here for the terms and conditions. Course Accreditation Accredited by Active IQ

A System Approach to Good Pharmacovigilance Practice
By Research Quality Association
Course Information In today's evolving landscape, pharmacovigilance (PV) systems face ongoing challenges due to global, national, and company-specific events. This course focuses on developing personnel equipped to navigate these complexities and improve the safe use of medicinal products. We emphasise continuous global thinking, communication, and strategic planning, ensuring adaptability across various levels. The course explores maintaining PV system functionality while adhering to regulatory requirements. Participants will apply European regulatory standards to enhance and sustain PV system effectiveness, contributing to improvement initiatives and ensuring operational integrity. The course covers PV system intricacies, regulatory compliance, and a 'systems approach' for auditors, quality assurance personnel, and PV practitioners. Through presentations and workshops, attendees gain insights into implementing and maintaining an effective PV system. Who should attend? Auditors Pharmacovigilance Quality System Managers Pharmacovigilance scientists The QPPV. Course benefits Throughout the course delegates will explore application of the legal requirements for the PV system and quality system and how to assure these systems. They will demonstrate their ability to contribute to: A systematic investigation of the pharmacovigilance system and its quality system Examination of how the pharmacovigilance system and quality system interact to achieve compliance. The risk-based approach to auditing the PV system and quality system The maintenance of 'inspection readiness' Explore how to investigate the complex PV system Discussions about how to monitor and maintain the PV system and assure compliance. Course Objectives Clarify what has to be done: Explore application of the legal requirements. Explore how to do what has to be done: Adopt a systemic approach to systematically investigate or implement and maintain the PV system and quality system Examine how a compliant PV system and a compliant quality system interact to achieve compliance with regulatory requirements for PV Explore how to investigate the complexity of the PV system. Discus how to identify what is missing or what needs to be improved: Discuss how to monitor and maintain the PV system and assure compliance. This course will assist delegates with: An understanding of key system principles, A practical approach to implementing, maintaining and monitoring the PV system and its quality system A procedure to share expertise to increase efficiency and confidence. This course is structured to encourage delegates to: Discuss and develop ideas - Share knowledge and experiences - Solve specific problems. By the end of the course delegates will be able to: Understand better the pharmacovigilance system, its quality system and how the components interact to achieve the objectives of pharmacovigilance Investigate, and analyse the pharmacovigilance system and to identify what is missing and what needs to be improved. Tutors Tutors will be comprised of (click the photos for biographies): Jana Hyankova Head of PV Department, IVIGEE Services a.s. Programme Please note timings may be subject to alteration. Day 1 08:30 Welcome, registration, course objectives and introduction to work groups Housekeeping notices, meet other delegates, explore how to work in your work group, course objectives. Clarify the definition and objectives of Pharmacovigilance. 09:30 The Regulatory Framework for Pharmacovigilance Identify the relevant regulations and directives. Explore GVP guidance, structure of the modules and standard format of each module. 10:00 The Pharmacovigilance System Exploration of how to organise what has to be done, communications. Exploration of the structures and processes for pharmacovigilance. 10:30 Break 11:00 Workshop 1 and Feedback Exploring an organisational model of the pharmaceutical company- cooperation between PV and other stakeholders. 12:00 The Quality System for pharmacovigilance Exploration of the structures, processes for the PV quality system and discussion of how it interacts with the pharmacovigilance system to meet the objectives of pharmacovigilance effectively and efficiently. 13:00 Lunch 14:00 Workshop 2 and Feedback The quality system puzzle. Explore the organisation of the PV quality system and how it interacts with the PV system. 14:30 The Quality System for pharmacovigilance Exploration of the structures processes for the PV quality system and discussion of how it interacts with the pharmacovigilance system to meet the objectives of pharmacovigilance effectively and efficiently. 15:00 Description of PV System 15:30 Break 15:30 Workshop 3 and Feedback The quality system puzzle Explore the organisation of the PV quality system and how it interacts with the PV system. 16:00 The Pharmacovigilance Safety Master File Construction of the Pharmacovigilance System Master File and its purpose. 17:00 Workshop 3 and Feedback Description of PV System. 18:00 End of Day Day 2 08:30 Drug Safety in the Clinical Trial Environment - Part 1 Information flow and responsibilities of the sponsor. 09:30 Workshop 4 and Feedback Drug Safety in the Clinical Trial environment: Information flow and responsibilities of the sponsor. 10:30 Break 11:00 Drug Safety in the Clinical Trial Environment - Part 2 Information flow and responsibilities of the sponsor. 12:00 Lunch 13:00 Workshop 5 and Feedback Drug Safety in the clinical trial environment: Information flow and responsibilities of the sponsor. 13:30 Processing of Safety Data Exploration of safety data processing, verification, validation, follow up, formatting and collation, reporting requirements, quality and data management. 15:00 Break 15:30 EudraVigilance Exploration of how EudraVigilance supports the PV system. 16:15 Signal Detection and Evaluation/Risk Benefit Assessment: Pharmacovigilance Risk Assessment Committee (PRAC): What is a signal? What are the regulatory requirements? How is signal detection and evaluation conducted? Qualitative and quantitative methods of signal detection. Risk benefit assessment. 17:00 Risk Management Plans A cornerstone of Pharmacovigilance safety communications, direct healthcare professional communication 18:00 End of Day Day 3 08:30 The Pharmacovigilance Risk Assessment Committee (PRAC) Exploration of how good practice is achieved. Composition, role and responsibilities. Examples of referrals. 09:15 Development Safety Update Reports (DSURs): Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 10:00 Periodic Safety Update Reports (PSURs)/Periodic Benefit Risk Evaluation Reports (PBRERs) Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 10:30 Break 11:00 Periodic Safety Update Reports (PSURs)/Periodic Benefit Risk Evaluation Reports (PBRERs) Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 12:00 Workshop 6 and Feedback To explore the compilation and submission of the PSUR. 13:00 Lunch 13:30 Role of the QPPV Exploration of the legal responsibilities of the QPPV and the MAH. 14:30 Break 15:00 Workshop 7 and Feedback To explore the challenges faced by the QPPV. 15:30 End of course Extra Information Face-to-Face Course Course material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device< Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking You will need a stable internet connection, a microphone and a webcam. CPD Points 23 Points Development Level Develop

Introduction to Computer Systems Validation
By Research Quality Association
Course Information Join our comprehensive three-day training programme tailored for individuals seeking a foundational grasp of computerised system validation. This course equips participants with essential knowledge to effectively validate systems within their respective organisations for utilisation in GxP (GLP, GCP, GMP, GDP, and GPvP) environments. Attendees will also gain proficiency in auditing validated computerised systems, ensuring compliance with pertinent GxP regulations. Commencing with an overview of regulatory prerequisites and the system life cycle, the course transitions into practical aspects, centered around validating computerised systems and conducting subsequent audits. Engage in a dynamic blend of presentations, interactive discussions, and hands-on practical workshops throughout the course. This course will provide delegates with an understanding of the computerised system validation process, including: Definition of end user requirements Risk management, including supplier assessment and techniques for audit planning Validation planning and reporting Linking system development with good business practices Formal testing and qualification Understanding of data integrity and security issues How to assess system validation documentation to verify compliance. Is this course for you? IT professionals new to implementing computerised systems into regulated environments Quality professionals who monitor or audit computerised systems System owners, end users, tester and project staff. Tutors Tutors will be comprised of (click the photos for biographies): Nichola Stevens Director and Principal Consultant, Nuncius Compliance Solutions Ltd Barry McManus Consultancy Partner, Empowerment Quality Engineering Ltd Programme Please note timings may be subject to alteration. Day 1 09:00 Welcome, Introduction and Course Objectives 09:45 Why Validate? Regulations and Guidance on Computerised System Validation Overview of the regulations and guidance applicable to CSV and their key expectations. 10:30 Break 10:45 The System Lifecycle The concept of the SLC and the key outputs from it. 12:00 Lunch 12:45 The Validation Process The approach to validation for different system types and a look at some of the key deliverables. 14:00 Project Introduction 14:15 Exercise 1 - User Requirements Capturing, agreeing and documenting the user requirements for a system. 15:15 Break 15:30 Exercise 1 - Feedback 16:00 Risk Management Risk management and its impact on validation. Identifying the deliverables required. Then group discussion on risk assessment for three systems. 17:00 Questions and Answers Answers to any outstanding questions from Day 1. 17:15 Close of Day Day 2 09:00 Supplier Assessment The different approaches to supplier assessment and the things to be considered when assessing a supplier. 10:15 Exercise 2 - Supplier Assessment Planning a vendor audit with a focus on the key validation deliverables. 11:00 Break 11:15 Exercise 2 - Feedback 11:45 Test Overview and Test Planning The different test phases, the purpose of each test phase and things to be considered when planning and reporting testing. 12:45 Lunch 13:30 Test Overview and Test Planning Continued. 14:15 Test Script Design, Execution and Review What a good test script looks like and the key things to consider when creating, executing and reviewing a test script. 15:30 Break 15:45 Exercise 3 - Creating a Test Script Create a test script based on user requirements created on Day 1. 17:15 Close of Day Day 3 09:00 Exercise 3 Feedback 09:30 Infrastructure Configuration and Qualification 10:30 Break 10:45 Validation Reporting Overview of the Validation Report and what should be included in it. 11:15 Maintaining the Validated State The procedures and records needed to ensure the system remains fit for purpose. 12:30 Lunch 13:15 Change Control Key concepts related to making changes to validated systems. 14:00 Data Integrity and Security How can we assure the integrity and security of our data. 15:15 Break 15:30 Course Objectives Summary and Panel Discussion A round up of key learning from the course. 17:00 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 19 Points Development Level Develop

CPD Accredited Botox & Dermal Filler Training
By The Angel Academy Of Teaching & Training
FOUR DAY ATTENDENCE AND TRAINING GUIDELINE: DAY ONE AND TWO - INTRODUCTION TO DERMAL FILLERS Day one Arrive and coffees (10.00) Registration, introduction and expectations (10.00-10.15) Structure of the training (10.15-10.30) Break (10.30-11.00) Lectures and interactive workshops / simulation (11.00 - 1230pm) Health and safety in the workplace Sharps injury and disposal The consultation process and prescriptions LUNCH (1300-1730) with a coffee break Basic life support Anaphylaxis - recognition and management Emergency kits - what it should contain and how to buy one Your doctors on call - how to contact our on call doctors for emergency advice How to use Hyalase safely - when to use it / recognise mechanism of action, how prescription in an emergency works and how to give the hyalase Practical and to include demonstration of Hyalase injection Our added benefits services for safety and convenience Day two Arrive and coffees (10.00) introduction and expectations (10.00-10.30) Structure of the training (10.30-11.00) Formal written examination covering key areas of THEORY for Dermal Filler injections: Anatomy, Physiology, Products and Complications. This will highlight early on if any important areas need to be covered in more detail for the students (11.00 - 1200) - Break for lunch - Practical session commences - (12.30 - 1800) - and in total on average we have scope for one model per 30 minutes on both of the Dermal filler days, so that’s a potential for 10 in total for a class size of maximum 4, which will give good hands on experience, as the way we train is to allow several people the opportunity to be involved with each patient - e.g. splitting into the phases of treatment, which allows the trainees to understand the concept of the treatment process. That would be - consultation, consent, marking up, readying equipment, performing the injection, providing advice and aftercare. DAY THREE AND FOUR BOTOX FOUNDATION COURSE Day three Arrive and coffees (10.00) introduction and expectations (10.00 – 10.30) Structure of the training (10.30 – 11.00) Formal written examination covering key areas of THEORY for Botox Application: Anatomy, Physiology, Products and Complications. This will highlight early on if any important areas need to be covered in more detail for the students (11.00 - 1200) - Break for lunch - Practical session commences - (12.30 - 1800) - and in total on average we have one model per 30 minutes on both the botox and days, so that’s a potential of 10 in total for a class size of 4, which will give good hands on experience, as the way we train is to allow several people the opportunity to be involved with each patient - e.g. splitting into the phases of treatment, which allows the trainees to understand the concept of the treatment process. That would be - consultation, consent, marking up, readying equipment, performing the injection, providing advice and aftercare. Day four Observed Treatment Process Examination The participants will be tested on the following key facets of safe practical care: Consultation process - rapport and understanding what the client wants Safe consent Marking and photographs Technical skill of injection Atercare provision and safety netting (eg if this happens do this / call me) 1 model will be provided for Botulinum (3 area) treatment and 1 - 2 clients for filler to ensure that each of the key anatomical areas covered are observed. Morning = Botulinum (0900 - 1230) Afternoon = Botulinum and Option Dermal fillers (1330 - 1630) Conclusion Candidates given session and refreshments and discussion regarding Case Studies and further support. (1700 - 1800)
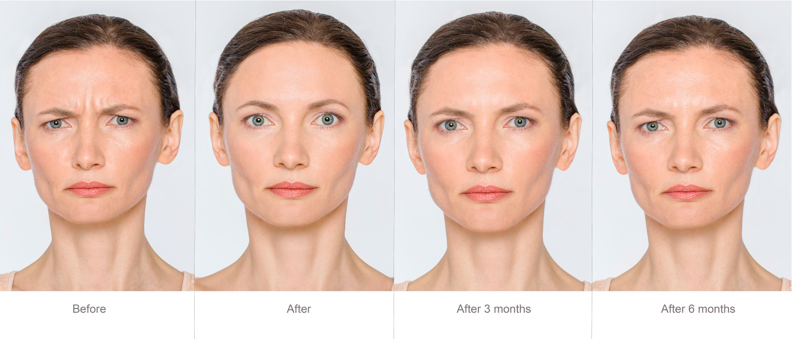
Auditing Computerised Systems
By Research Quality Association
Course Information Join our comprehensive three-day programme designed as an invaluable external training opportunity for auditors, audit programme managers, and individuals subject to audits. This course is tailored to foster a deep understanding and cultivate essential skills for auditing the validation of computer systems intended for GxP environments (GLP, GCP, GMP, GDP, GPvP). Commencing with an overview of regulatory prerequisites and the system life cycle, the course swiftly transitions to focus on the pragmatic aspects of auditing computer system validation. Experience a blend of presentations, interactive discussions, and immersive practical workshops throughout the duration of the course. Delegates will benefit from practical examples of how to understand the framework of applicable regulations and guidance. Apply risk management techniques to audit planning Plan and conduct computerised system audits Assess system validation documentation to verify compliance Evaluate data integrity and security issues Prepare for regulatory inspection. The course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Understand the vulnerabilities of computerised systems Learn how to create a compliance checklist Link system development with good business practice. Is this course for you? Auditors Audit programme managers Individuals subject to audits. Tutors Tutors will be comprised of (click the photos for biographies): Nichola Stevens Director and Principal Consultant, Nuncius Compliance Solutions Ltd Barry McManus Consultancy Partner, Empowerment Quality Engineering Ltd Programme Please note timings may be subject to alteration. Day 1 09:00 Welcome and Introductions 09:45 Why We Validate and Regulatory Trends 10:30 Break 10:45 Audit Overview, High Level Process and Scheduling 11:30 System Lifecycle 12:30 Lunch 13:15 Exercise 1 - Audit Scheduling 14:45 Exercise 1 - Feedback 15:15 Break 15:30 Validation Deliverables 16:30 Risk Assessments 17:30 Close of Day 1 Day 2 09:00 Supplier Assessment 10:30 Break 10:45 Exercise 2 - Planning a Supplier Audit 12:00 Exercise 2 - Feedback 12:30 Lunch 13:15 Exercise 3 - Auditing a Computerised System Validation Package 15:30 Break 15:45 Exercise 3 - Feedback 16:30 Change Control 17:15 Close of Day Day 3 09:00 Infrastructure Qualification 09:45 Maintaining a Validated State - Operational Processes 11:00 Break 11:15 Exercise 4 - Auditing Systems in Operational Use 12:45 Lunch 13:30 Exercise 4 - Feedback 14:15 Exercise 5 - Auditing Trail Review 15:30 Break 15:45 Exercise 5 - Feedback 16:15 Course Objectives Summary and Any Additional Questions 16:45 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 19 Points Development Level Develop

Search By Location
- Nursing Courses in London
- Nursing Courses in Birmingham
- Nursing Courses in Glasgow
- Nursing Courses in Liverpool
- Nursing Courses in Bristol
- Nursing Courses in Manchester
- Nursing Courses in Sheffield
- Nursing Courses in Leeds
- Nursing Courses in Edinburgh
- Nursing Courses in Leicester
- Nursing Courses in Coventry
- Nursing Courses in Bradford
- Nursing Courses in Cardiff
- Nursing Courses in Belfast
- Nursing Courses in Nottingham