- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
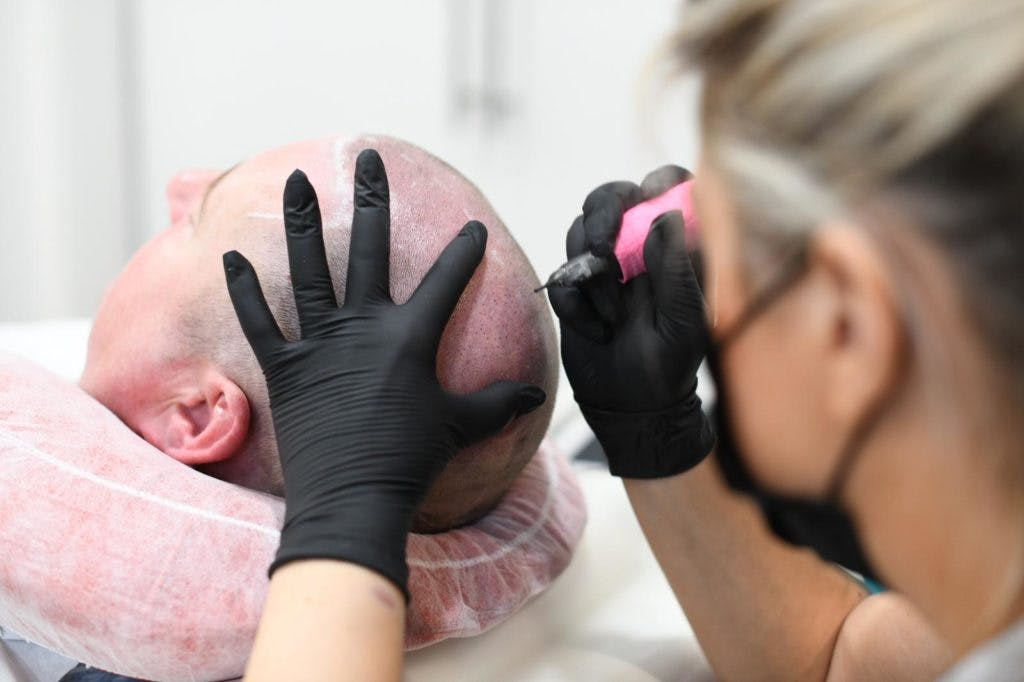
Scalp Micropigmentation Training Course
By Cosmetic College
Scalp micropigmentation, otherwise known as SMP, is applying pigment to the epidermal level of the scalp to replicate the natural appearance of hair follicles. Similar to tattooing, the ink used during scalp micropigmentation permanently marks the scalp, giving the look of a full head of hair that has been shaved or adding density to hair that may be thinning. For many people suffering from hair loss, Alopecia or scarring, this cosmetic treatment offers a life-changing, viable and long-term solution. As a practitioner, scalp micropigmentation requires in-depth training, specialised equipment and extensive knowledge and skill. The process consists of multiple sessions in which carefully selected pigment is implanted into the scalp using specialised needles. One of the great things about doing micropigmentation training is that it does not require you to have any medicine or surgery background. You can undertake micropigmentation courses and become proficient in the technique in a short time. This means you can start practising extremely quickly after deciding to join the industry. Course Prerequisites This course is suitable for those with no prior experience and is designed to provide the student with the ability to seek employment or start their own business upon qualification. Course Pre Study Before beginning practical training, students are required to complete: 30 hours of easy to access e-learning A series of online assessments Course Structure This is a 1 days intensive theory and practical sessions. All courses are kept intimate with a maximum of 4 students per course. Areas of study: The fundamental theory of cosmetic tattooing Anatomy and Physiology Health and Safety Colour Theory Client Assessment and Suitability Legal requirements, obtaining consent with consultation techniques and documentation Clinical setup procedures Pre-treatment drawing techniques Practical technique sessions on practice materials and model clients. Professional live demonstrations Industry leading pre and post treatment care for your clients Practical areas included in depth within the course are: Proper hairline design Blending techniques Sanitation and sterilisation Proper station set up Skin colour matching analysis Needle specification
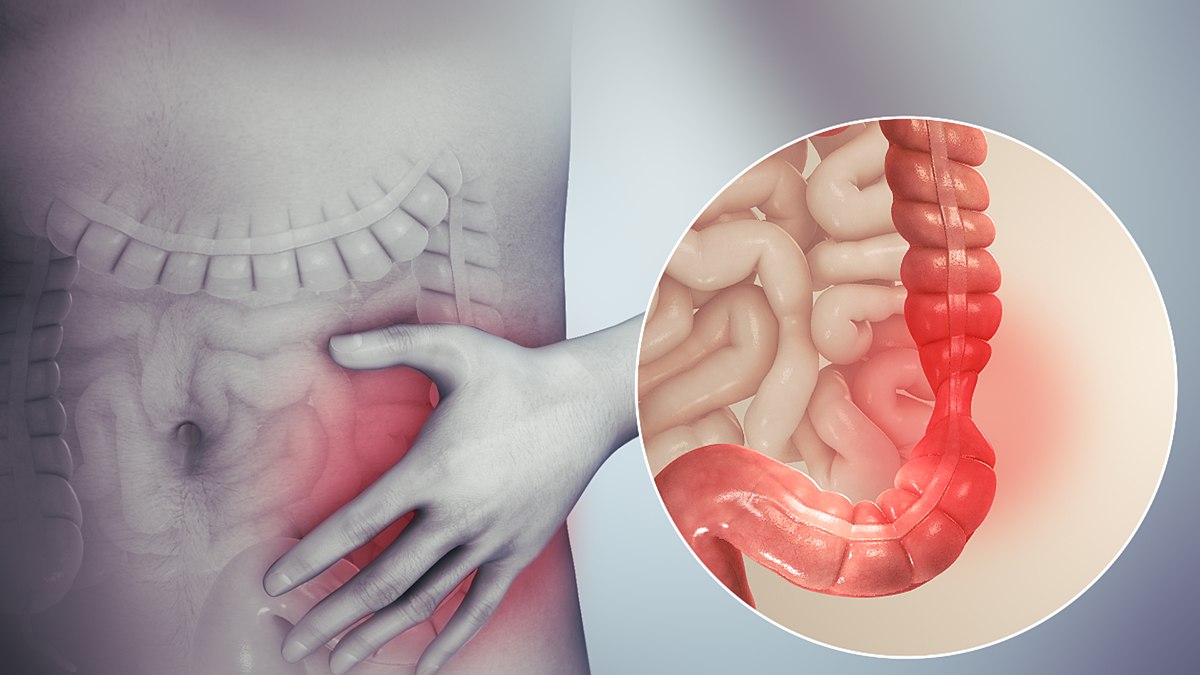
An Understanding of Bowel Care
By Guardian Angels Training
Gain comprehensive knowledge and practical skills for safe and effective bowel care management with our "An Understanding of Bowel Care Techniques" course. Ideal for healthcare professionals seeking to promote patient comfort and prevent complications.
Digital Brows Training | Fundamental Beginners PMU Training - 1-2-1 Private Training
By ID Liner | Permanent Makeup Training & Supplies
students learn a variety of different brow tattooing techniques, so we will spend two days learning the shaded brow effects possible with a digital device and three days focused on our most-requested Hairstroke Brows.

GLOSS & GO™ Lip Blush Training | Fundamental Beginners PMU Training - Small Group Learning
By ID Liner | Permanent Makeup Training & Supplies
On successful completion of the ID Liner Gloss & Go™ Lip Blush training course, students will not only be given the skills and tools to offer this incredibly popular treatment to their clients but will also be permitted to advertise the trademark, giving them an edge in a competitive market.

Permanent Eyeliner | Fundamental Beginners PMU Training - 1-2-1 Private Training
By ID Liner | Permanent Makeup Training & Supplies
The objective of the ID Liner Permanent Eyeliner fundamental course is to teach you how to achieve this look for your clients. It is the perfect solution for clients who struggle to draw on their own eyeliner or who just want an expertly enhanced look 24/7

Train the Trainer / Instructor Course in Nasogastric Tube Insertion and Feeding
By Guardian Angels Training
Gain comprehensive knowledge and practical skills for safe and effective nasogastric tube insertion and feeding techniques with our "Promoting Best Practice in Nasogastric Tube Insertion and Feeding Tuition" course. Optimise patient safety, comfort, and outcomes with evidence-based best practices.

Working with Children and the Foundations of PBS
By Guardian Angels Training
Enhance your skills in promoting positive behavior and creating supportive environments for children with our PBS course. Evidence-based practices and collaboration emphasised.

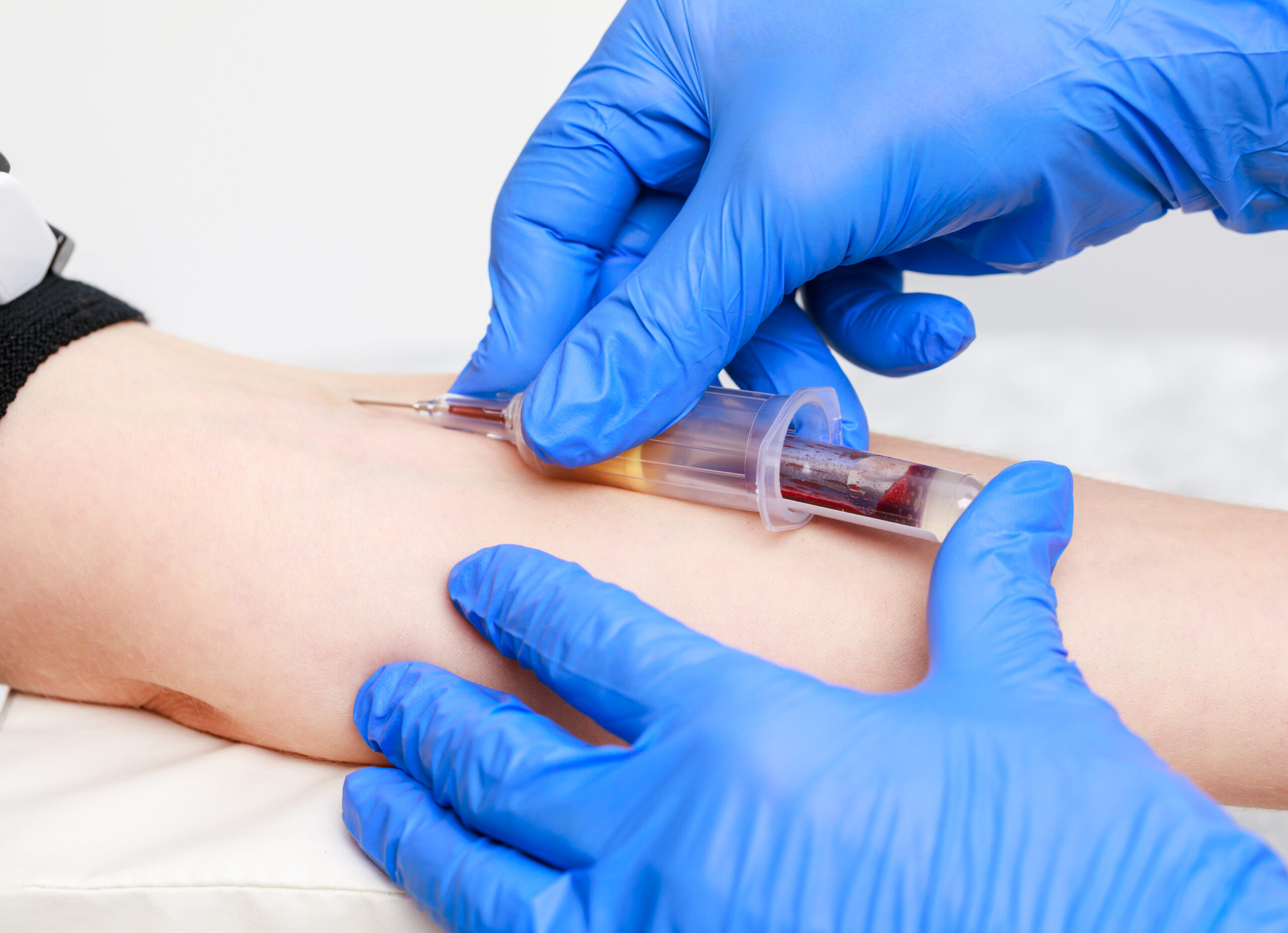
An Understanding of Venepuncture (Adult or Child as applicable)
By Guardian Angels Training
Gain comprehensive knowledge and practical skills for safe and effective venepuncture procedures in adult patients with our "Understanding Adult Venepuncture Techniques" course. Perfect for healthcare professionals seeking to confidently perform venepuncture with accuracy and patient comfort.
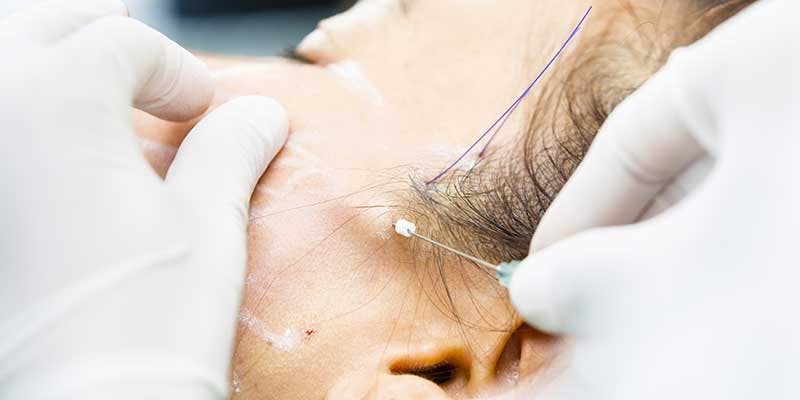
PDO COG Thread Lifts Training
By Cosmetic College
The 'No Blade Facelift' is the new trend made popular by numerous celebrities recently and it is an excellent and effective non-surgical technique to lift and tighten skin. In the right hands, it has the potential to re-define facial contours and induces collagen production. The treatment forms part of an integrated support structure for the tissue of the face by encouraging natural collagen synthesis with immediate results that peak at 6 months and last between 2-3 years. âOn this course, we aim to help you master a technique that will set you apart from most routine cosmetic treatment providers and enable you to step into the future of advanced cosmetic procedures. We will cover all you need to know in order for you to treat your patients confidently and safely; including anatomy, use of local anaesthetic, consultation and assessment, managing complications and aftercare. You will perform this procedure on live models under the supervision and guidance of highly experienced aesthetic practitioners Course prerequisites This course is suitable for those with or without a medical background. It is designed to provide the student with the ability to seek employment or start their own business upon qualification. At a minimum, students will be required to be qualified for at least one of the following: Medically qualified as a nurse, doctor or dentist with current registration with the NMC, GMC or GDC. NVQ Level 3 in Beauty Therapy, ITEC or HND 12 months of needling experience 6 Months of micropigmentation experience and Anatomy & Physiology Level 3 If your qualification does not appear above, we offer a fast track access course for those completely new to the industry. Course agenda Background of PDO Threads Health & safety In-depth anatomy and physiology Emergency protocols Product knowledge Sourcing clinical oversight (Prescriber) Complications prevention Client suitability Equipment use Needle stick injury protocol Pain management with the use of injectable anaesthetic Adverse effects Complications management Emergency Protocols Anaphylaxis Aftercare Consent forms Consultation process Client selection Live demonstrations Live model experience Recommended treatment charges Insurance Legalities Advance your training with our complete PDO Threads training package Take your training to the next level by enrolling on our complete PDO Thread training package. Included within this package: PDO COG Threads PDO Mono Threads Fox Eye Thread Lifts Pixie Nose Tip Lifts Check out the package here
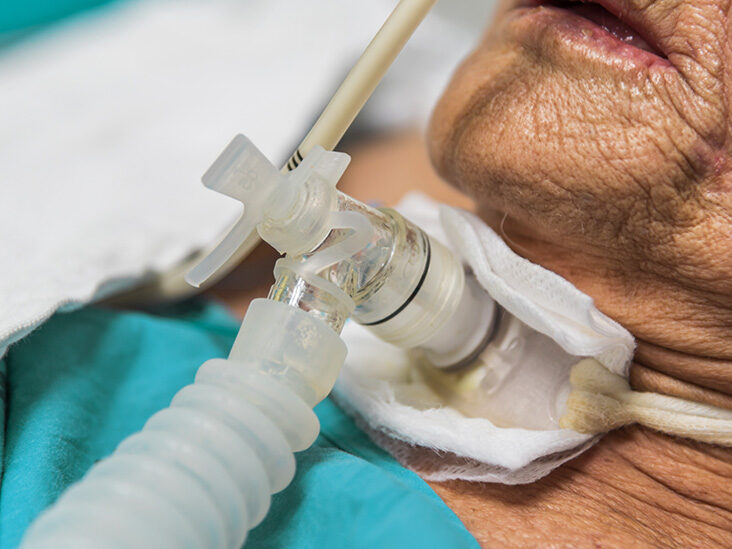
An Understanding of Tracheostomy Care
By Guardian Angels Training
Gain comprehensive knowledge and practical skills to provide safe and effective care for tracheostomy patients with our "Understanding Tracheostomy Care" course. Learn the management and maintenance of surgical openings in the trachea to facilitate breathing.
Search By Location
- Consent Courses in London
- Consent Courses in Birmingham
- Consent Courses in Glasgow
- Consent Courses in Liverpool
- Consent Courses in Bristol
- Consent Courses in Manchester
- Consent Courses in Sheffield
- Consent Courses in Leeds
- Consent Courses in Edinburgh
- Consent Courses in Leicester
- Consent Courses in Coventry
- Consent Courses in Bradford
- Consent Courses in Cardiff
- Consent Courses in Belfast
- Consent Courses in Nottingham