- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
NTP and PTP training course description This course looks at timing and synchronization as provided by NTP and PTP. Hands on sessions primarily involve using Wireshark to analyse the protocols. What will you learn Recognise the importance of timing and synchronisation. Explain how NTP works. Explain how PTP works. NTP and PTP training course details Who will benefit: Anyone using NTP or PTP but particularly relevant for those in the broadcast industry. Prerequisites: TCP/IP foundation for engineers Duration 1 day NTP and PTP training course contents Introduction Clock drift. Timing and synchronization. Importance in computing. Importance in broadcasting. NTP NTP versions, v0 to v4. Architecture. The intersection algorithm. Accuracy. Clock strata, Stratum 0, 1, 2 and 3. Protocol modes. Hands on NTP configuration More NTP NTP packet header. Timestamps. Variables in the header. Clock synchronization algorithm. SNTP. The Windows Time service. Hands on Wireshark and NTP analysis. PTP PTP v2, IEEE 1588. Architecture. Accuracy. Synchronization with PTP. Offset and delay. Hands on Analysing PTP packet flows. More PTP Ordinary clocks, boundary clocks, masters and grandmasters. PTP specific switch types. Hardware time stamping. SMPTE ST2059-2. PTP packet header. PTP domains. Best master clock algorithm. Hands on More Wireshark and PTP.

Quality Assurance for Good Laboratory Practice
By Research Quality Association
Course Information A must-have programme for Quality Assurance auditors stepping into or honing their role within a Good Laboratory Practice (GLP) environment, this course offers invaluable, expert guidance for crafting a robust and efficient GLP audit programme. What will I learn? A solid regulatory foundation underpinning quality assurance activities Clarity on the roles of Quality Assurance, management, and study director within the framework of Good Laboratory Practice principles Enhanced efficacy in inspections and audits Heightened compliance with Good Laboratory Practice standards for your facility Unique insights into governmental monitoring activities within the GLP sphere. This course is structured to encourage delegates to Discuss and develop ideas Solve specific problems Examine particular aspects of GLP. Tutors Tutors will be comprised of (click the photos for biographies): Cate Ovington Director, The Knowlogy Group Ltd Jane Elliston Senior Quality Assurance Auditor, Battelle UK Shona Ross Head of QA, Tower Mains Ltd Programme Please note timings may be subject to alteration. Day 1 09:00 Welcome and Introductions 09:15 Good Laboratory Practice Standards and Regulations An insight into the background and history of Good Laboratory Practice. 09:45 Principles of Quality Assurance What is the role and responsibilities of QA in GLP. Maintaining the independence of QA and what is an audit. 10:30 Break 10:45 Standard Operating Procedures GLP requirements and QA involvement. 11:30 Study Plans GLP requirements and QA involvement. 12:05 QA Programme Risk based programme, what are study, process and facility audits. 13:00 Lunch 14:00 Inspections Attitudes, techniques and attributes. 14:40 Workshop 1 - Facility and Process Inspections An exercise in inspection planning and preparation for inspections. 15:15 Break 15:30 Workshop 1 - Feedback 15:45 The Auditor and Audit Conduct Attitudes, attributes and techniques. 16:30 Panel Session An opportunity for delegates to put questions to the panel of speakers. 17:15 Close of Day Day 2 09:00 Workshop 2 - A Mock Audit 10:45 Break 11:00 Workshop 2 - Feedback 11:30 Auditing the Study Report Techniques and methods for the QA audit of the study report. 12:00 Record Keeping and Data The impact of GLP on data and records management. 12:40 Lunch 13:25 Data Integrity A look at the OECD GLP guidance document; the expectations of the regulators and the involvement of QA - Where QA adds value. 14:15 Workshop 3 - Amendments to Study Plan and Deviations from the Plan What are they? What is the difference between them? How are they controlled? 15:00 Workshop 3 - Feedback 15:15 Break 15:30 Regulatory Compliance GLP Monitoring Authority monitoring for compliance with Good Laboratory Practice. 16:15 Panel Session An opportunity for delegates to put questions to the panel of speakers. 16:45 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Learn

Implementing Good Clinical Laboratory Practice
By Research Quality Association
Course Information Join our comprehensive course, meticulously designed to equip individuals implementing Good Clinical Laboratory Practice (GCLP) within laboratories handling samples from clinical trials. Delve into the current regulatory landscape governing laboratory work supporting clinical trials, referencing key guidelines such as the ICH Guideline for Good Clinical Practice, the Clinical EU Trials Directive, relevant regulations, and leveraging insights from the RQA guidance document on GCLP. Is this course for you? This course is tailored for laboratory managers, analysts, investigators, trial coordinators, monitors, and auditors operating in diverse settings such as pharmaceutical company laboratories, central laboratories, contract research organisations, hospital laboratories, clinics, and investigator sites. This course will give you: Guidance on effectively interpreting and applying GCLP within the broader framework of Good Clinical Practice (GCP) Insight into the seamless integration of GCLP within clinical programmes (GCP) Practical strategies for implementing GCLP in the nuanced environment of clinical research laboratories The chance to update your knowledge with the latest interpretations and guidance on clinical laboratories by the UK Medicines and Healthcare Products Regulatory Agency (MHRA) Access to a seasoned panel of speakers with extensive expertise A unique opportunity to deepen your understanding of GCLP's application across diverse scenarios. Engage in: Lively discussions to foster ideas Problem-solving sessions targeting specific challenges Detailed exploration of specific aspects within the realms of GCP and GCLP. Tutors Tutors will be comprised of (click the photos for biographies): Vanessa Grant -, - Louise Handy Director, Handy Consulting Ltd Tim Stiles Consultant, Qualogy Ltd Programme Please note timings may be subject to alteration. Day 1 08:50 Registration 09:00 Welcome and Introduction 09:20 Good Clinical Practice and the Requirements of Good Clinical Laboratory Practice A review of Good Clinical Practice and its requirements for the laboratory analysis of samples from a trial. The thought processes behind the development of Good Clinical Laboratory Practice, its objective, scope, interpretation and application are explained. 10:00 Safety and Ethical Consideration Informed consent, confidentiality, expedited reporting, blinding and unblinding and serious breaches of the GCP are discussed. 10:40 Break 10:55 Organisation and Personnel Responsibilities within GCP and the Laboratory The responsibilities of key functions that should exist within a clinical laboratory including personnel records of training and competence are discussed. 11:30 Staff Training and Training Records Personnel records of training and competency assessments are discussed. 11:45 Laboratory Facilities, Equipment and Materials Suitable facility design, organisation and operation will be discussed. The calibration, validation and maintenance of equipment used in the conduct of sample analysis are examined, as are the suitability of materials and the identification and labelling of reagents and solutions. 12:30 Lunch 13:15 Workshop 1 - Facilities, Equipment and Responsibilities Some practical problems with regard to the facilities, equipment and responsibilities are explored. 13:45 Workshop 1 - Feedback 14:15 Computer Systems Validation Systems, including computerised systems, used in the analysis, collection and reporting of results should be appropriately tested, operated and controlled. What this means in practice is discussed. 14:45 Trial Protocols, Analytical Plans During this session we examine the purpose, content, control and change of these important documents. 15:30 Break 15:45 Workshop 2 - SOPs, Clinical Protocols, Analytical Plans and Validation The practicalities of managing and documenting the planning phase of analytical work on a trial are explored along with computerised system validation. 16:30 Workshop 2 - Feedback 17:00 Close of Day Day 2 09:00 Conduct of the Work and Quality Control Many of the issues that surround the conduct of sample collection, shipment, storage, analysis and management of Analytical Methods are discussed. This includes the quality control of the assay that may be employed and Quality Control checks. 10:00 Deviation Management The expectations around deviations and CAPA are discussed. 10:15 Workshop 3 - Conduct of the Work and Quality Control Practical work conduct and quality control issues are explored. 10:45 Break 11:00 Workshop 3 - Feedback 11:30 Source Data, Data Integrity, Records and Reports The creation and subsequent management of source data and records, data integrity, are discussed, together with the process of reporting analytical results. 12:10 Workshop 4 - Data, Records and Reports Practical problems with data, records and reports are investigated. 12:45 Lunch 13:30 Workshop 4 - Feedback 14:00 Quality Audit The requirements for and purpose of quality audits are discussed. The difference between quality audit and quality control are explained along with the role of the quality audit staff and their interaction with the analytical project managers, laboratory management and study staff. 14:40 Risk Management How should we assess risk and how can we use the process to assist in evaluation of audit findings. 15:15 Break 15:30 Regulatory Inspection The conduct of regulatory inspections and current expectations of the inspectors. Preparation for inspections and conduct during them will be discussed. 16:00 Panel Session This panel session will address any outstanding issues raised by the delegates. 16:15 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Develop

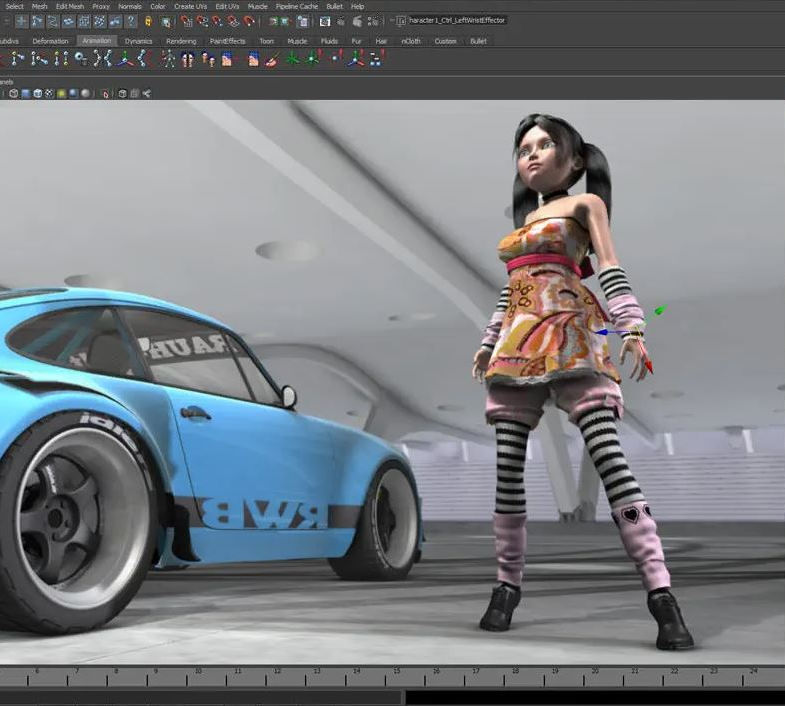
Autodesk Maya Basics to Advanced Level
By London Design Training Courses
Why Learn Autodesk Maya Basics to Advanced Level Course? Course info. Autodesk Maya is one of the best tools for 3D animation and visual effects. Learn Maya with our training courses covering sectors like Architecture, Games & Films, Animation, and Media. London Design Training Courses empower you to master Maya, transforming your 3d skills.  Duration: 40 hrs Method: 1-on-1. Schedule: Tailor your own schedule by pre-booking a convenient hour of your choice, available from Mon to Sat 9 am and 7 pm. Experience advanced-level Maya training covering essential aspects such as modeling, UV mapping, texturing, lighting, camera movement, and rigging. The course is tailored to your specific requirements and work preferences. You can discuss your learning goals with our trainer, and we will suggest a syllabus that meets your needs. Flexible Training Options: Choose in-class training at one of our UK center or attend live online sessions. Certified Tutors and Authoritative: London Design Training Course has all Autodesk Authorized Trainers. Hands-on Learning Approach: The training is practical and hands-on, combining theory and step-by-step demonstrations. You'll have ample time to practice techniques on your computer with Maya, and you can keep all the files you create. Compatible with Windows and Mac: Maya training is available for both Windows and Mac users, with options to suit any recent version of Maya. Maya Basic to Advanced Course Duration: 40 hours Course Description: In this course, you will learn the fundamental and advanced skills to create complex 3D models and animations using Autodesk Maya software. You will gain knowledge of the Maya interface, tools, and workflow. You will learn the essentials of modeling, texturing, rigging, animating, and rendering 3D models. You will also learn how to create advanced special effects and understand advanced modeling techniques. I. Introduction to Maya (3 hours) Overview of Maya and its Uses Maya interface and basic tools Navigation and viewport controls Creating and saving projects II. Basic Polygon Modeling (6 hours) Polygon modeling basics Creating basic shapes and objects Editing and modifying objects Creating complex objects with extrusions and bevels Creating organic shapes with NURBS III. Intermediate Modeling (6 hours) Advanced polygon modeling techniques Creating complex models with Booleans and deformers Creating and modifying curves and surfaces Creating organic shapes with sculpting tools Creating architectural models IV. Texturing and Materials (6 hours) Introduction to texturing Creating and applying materials Texture mapping and UV unwrapping Creating realistic materials with shaders Painting textures with the 3D paint tool V. Lighting and Rendering (6 hours) Basic lighting techniques Advanced lighting techniques Creating realistic lighting environments Setting up a camera and creating a composition Rendering still images and animations Output options and file formats VI. Animation (9 hours) Introduction to animation Keyframe animation and animation curves Creating and editing animation clips Rigging and animating a simple character Creating and editing motion paths and animation layers Creating complex character rigs Creating lip sync and facial animation Creating realistic animation with dynamics and simulations Cloth Animation Ncloth VII. Rigging (4 hours) Introduction to rigging Creating joints and skeletons Binding skin to joints and creating weight maps Creating simple rigging systems and rigging a character Creating complex rigging systems VIII. Special Effects (4 hours) Particle systems and dynamics Creating and manipulating fluids and fire effects Creating and editing special effects like explosions and smoke Creating advanced simulations with nCloth and nParticles Paint effects Mash Networks and Mash Animation IX. Advanced Rendering Techniques (2 hours) Render layers and passes Global illumination and ambient occlusion Mental Ray rendering and settings X. Conclusion and Next Steps (1 hour) Review of course content Tips for further learning and resources Q&A; and feedback Note: The above course outline is just a suggestion, and the course content and duration can be adjusted according to the needs and level of the learners. Proficiency in Advanced Maya Techniques: Participants will gain advanced skills in various aspects of Maya, including modeling, UV mapping, texturing, lighting, camera movement, and rigging. Tailored Training: The course is customized to meet the specific requirements of participants, focusing on their preferred techniques and work type. Hands-On and Practical Experience: The training is practical and hands-on, allowing participants to practice techniques on their own computers with Maya. Versatility in Operating Systems: Participants will be equipped to use Maya on both Windows and Mac systems. Accredited Certification: Upon successful completion, participants will receive an e-certificate, accredited by Autodesk Certified instructor, confirming their achievement in the Maya training course. Post-Course Support: After the training, participants are entitled to 30 days of email support from their Maya trainer, ensuring assistance with any post-course questions or issues. Up-to-Date and Relevant Learning: The training can be based on any recent version of Maya, providing participants with up-to-date knowledge and skills.
Good Laboratory Practice for Study Directors, Principal Investigators, Study Staff and Management
By Research Quality Association
Course Information Embark on our GLP course offering extensive guidance and pragmatic support tailored for individuals serving as Study Directors or Principal Investigators overseeing non-clinical safety studies on pharmaceuticals, agricultural, and industrial chemicals within the realm of Good Laboratory Practice (GLP). This comprehensive programme extends its benefits to study staff and management operating in GLP-compliant environments. The course extensively covers the current OECD GLP Principles and UK GLP legislation, while also referencing international standards, regulations, and guidelines pertinent to the field. Benefits of this course: Practical help and guidance on the interpretation and application of GLP An opportunity to update your knowledge of GLP with the current interpretation of requirements Access to an experienced panel of speakers Information on how other organisations address GLP issues An opportunity to improve your understanding of the GLP requirements as they are applied in different situations. This course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Examine particular aspects of GLP Learn from the experience of others. Tutors Tutors will be comprised of (click the photos for biographies): Tim Stiles Consultant, Qualogy Ltd Tony Woodall Head of Quality Assurance, Alderley Analytical Gill Armour Study Monitor Team Leader, AstraZeneca Jane Elliston Senior Quality Assurance Auditor, Battelle UK Vanessa Grant -, - Jeanet Logsted CEO, Scantox Programme Please note timings may be subject to alteration. Day 1 09:00 Registration 09:15 Welcome and Introductions 09:35 Development of Good Laboratory Practice A review of the history of GLP, its current scope and application, with a synopsis of current European and international standards. 10:05 Roles and Responsibilities The responsibilities of study director, test facility, management and study staff in the conduct of a GLP study. 10:45 Break 11:00 The Roles and Responsibilities of the Study Director and Test Facility Management The role of the study director in the management and control of a study, as defined by GLP, and management's roles are explored. 11:45 Multi-site Studies What is a multi-site study and when should such concepts be applied on a study. The role of the study director and principal investigator in the planning, conduct and reporting of multi-site study are explored. 12:30 Study Plan (Protocols) GLP requirements for the preparation of a study plan, content, authorisation, amendments and deviations are discussed. 13:00 Lunch 13:45 Workshop 1 - The Study Plan Some practical problems with study plans and amendments explored. 14:45 Workshop 1 - Feedback 15:00 Standard Operating Procedures The control, content and authorisation of SOPs and the principles behind the practice. 15:30 Break 15:45 Workshop 2 - Practical Study Conduct Problems Dealing with practical problems encountered during the conduct of studies. 16:40 Workshop 2 - Feedback 17:15 Close of Day Day 2 09:00 Questions and Answers Discussion of issues raised by course delegates. 09:20 Quality Assurance The interactions between QA, management, study director and principal Investigator are discussed as is QAs role when conducting a multi-site study. 10:00 The Final Report The content of the final report and the role of those involved in its preparation and approval. Specific reporting requirements when conducting a multi-site study are also explained. 10:30 Break 10:45 Workshop 3 - Final Report Problems Practical problems of report preparation including compliance statements. 11:30 Workshop 3 - Feedback 12:00 Management of Raw Data and Records A view on how records and materials are managed and archived in compliance with GLP. 12:45 Lunch 13:30 Workshop 4 - Data and Sample Management Issues Dealing with data and sample management issues. 14:15 Workshop 4 - Feedback 14:45 Regulatory Inspection Government monitoring for compliance with Good Laboratory Practice. 15:15 Panel Session This panel session will address any outstanding issues raised by delegates. 15:45 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Develop

Effective Presentation Skills: In-House Training
By IIL Europe Ltd
Effective Presentation Skills: In-House Training In today's results-oriented, global working environment, the ability to create and deliver presentations effectively is a necessary skill set for people at all levels of an organization. Regardless of your role, it's important to know how to synthesize your ideas into a coherent and focused narrative, add visuals that support and reinforce your message, and deliver it in a way that resonates with your audience. In this highly interactive course, we will unpack and practice some of the tools and techniques used by top speakers and influencers all over the world. In this engaging two-day course, you will plan, write, refine, practice, and deliver a presentation to the class. Your presentation will be filmed on both days, and you will leave the course with a flash-drive copy of your videos; participants of the virtual classroom workshop should be prepared to present via webcam. In addition to discovering and enhancing your own personal delivery style, you will learn how to create an overarching goal for your presentation and then organize and structure it for maximum impact. You'll gain insight into how to anticipate your audience's needs and tailor the content and delivery in a way that connects with them and sustains their attention and engagement. You will also learn skills that will help you control nervous energy, remain focused on and attuned to your audience, improvise under pressure, deal effectively with questions, and build a compelling call to action. What you will Learn At the end of this program, you will be able to: Construct an effective presentation goal statement, opening, body, and closing that connect with an audience Analyze an audience's needs and style preferences, including relevant DiSC®-related elements Deliver a complete criteria-based presentation that will persuade others Align usage of visuals as well as verbal and non-verbal techniques to maximize the impact of your presentation Getting Started Introductions and social agreements Course structure Course goals and objectives Opening activities Planning and Organizing Video: 'The Art of Misdirection' Setting your presentation goal Writing a goal statement Analyzing your audience Applying the 'reality' test Creating and strengthening supports Structuring your presentation 5 components of an effective opening Presentation body Presentation closing Write your presentation opening Audience Analysis Video: 'How to Tie Your Shoes' Everything DiSC® introduction Audience DiSC® Styles Analyzing your audience Further audience analysis Effective Delivery Delivery challenges: virtual and in-person Keeping your audience engaged Your body as your instrument Verbal / paraverbal elements Body stance and nonverbal communication What are your 'tells?' Controlling nervousness Staying attuned to your audience Responding to questions Review and edit your opening Deliver your opening Visuals and Enriching Elements Using images in your presentation Guidelines for visual composition Using questions to engage your audience The power of the pause Practicing and Applying What You've Learned Preparation Delivery Feedback Opportunity to put into practice the program content and receive a video copy Summary and Next Steps What did we learn and how can we implement this in our work environment? Your personal action plan

Internet of Things training course description A concise overview course covering The Internet of Things and the technologies involved. Particular emphasis is placed on the high level architecture of IoT and the benefits achievable. What will you learn Describe the structure of the IoT List the technologies involved in IoT. Explain how IoT works. Internet of Things training course details Who will benefit: Non-technical staff working with IoT. Prerequisites: None. Duration 1 day Internet of Things training course contents What is IoT The Internet, what is IoT? IoT and M2M, IoT technologies, IoT architecture. Wired and wireless communication. IoT applications; Smart houses, smart cities, smart cars, wearable, environment, other domain specific IoTs. IoT architecture Physical objects, virtual objects, cloud computing, data capture, communications. Big data. Components Hardware, sensors, actuators, chips, firmware, embedded systems. Open source platforms. Power options: Battery, solar, PoE. IoT communication RF, ZigBee, Bluetooth, Bluetooth LE, RFID, WiFi, 802.11ah, mobile technologies. Wired. Arduino (as an example) Microcontrollers, the platform, development, Arduino software, reading from sensors, I2C, SPI. Arduino and the Internet, HTTP, WiFi, GSM. The cloud and IoT: Pachube, nimbits, ThingSpeak Security in IoT Authentication, Encryption, secure booting, firewalls.

Work Breakdown Structures
By IIL Europe Ltd
Work Breakdown Structures It's amazing how often project managers begin the project planning process by making an outlined list of every task they believe will be required to complete a project and then proclaim they have created the work breakdown structure (WBS) for the project. The result is a list of hundreds, or even thousands of tasks, many of them having durations of a few days or a few hours. Essentially, what they have done is create a 'to do' list, which they then use as a 'checklist' to measure progress. This approach leads to, and even encourages, micromanagement of the resources working on the project without consideration of more critical aspects of project management such as: requirements management, risk management, procurement management, estimating, scheduling, executing, and controlling. Further, it makes it impossible to see the big picture, at levels of detail, in keeping with the needs of sponsors, clients, project and functional managers, team leaders, and project performers. Join us for this exciting program and learn how to use the WBS to make better-informed business decisions. What You Will Learn You will learn how to: Describe the need for a project WBS Describe the WBS role in the project Gain practical experience in the development, decomposition, and use of the WBS Determine the appropriate level of detail in the WBS. Explain how the WBS integrates with project requirements, risk, procurement, estimating, scheduling, and overall project execution. Provide the basic tools to enhance efficient re-use of key information in your future projects Foundation Concepts Key definitions History of the WBS Importance of the WBS Overall structure Terminology Other breakdown structures WBS tools WBS & Scope Project scope management processes Specification of the project objectives WBS design based on project deliverable WBS decomposition process and 'The 100% rule' Work Packages and Control Accounts WBS & Risk Risk management planning and WBS Risk identification to enhance the WBS Risk analysis and the WBS Risk responses and updating the WBS Implementing risk response and Monitoring risks and the WBS WBS & Estimating Use of WBS in the estimating process Components and work packages Sizing and algorithmic estimates WBS & Scheduling Component Scheduling - High-Level Milestones WBS activity decomposition WBS elements dependencies Work Package Level Schedules Responsibility assignment matrix WBS & Execution and Control Earned Value Management and tracking of work performance Progress reports, forecasts, and corrective and preventive actions used to manage work performance Necessary information to close out a project

Awareness of First Aid for Mental Health
By Madeleys First Aid Plus
RQF level 1 Awareness of First Aid for Mental Health Each year approximately 1 in 4 people in the UK will experience a mental health condition and at least 1 in 6 employees experience common mental health problems in the workplace. Research has shown that work is the biggest cause of stress which can stop people performing at their best. Mental health conditions are often hidden due to stigma and fear of discrimination and research has shown that a culture of fear and silence around mental health is costly to employers. The HSE guidance 'First aid needs assessment’ refers to mental health in the workplace. This 4-hour qualification provides learners with the knowledge to recognise a range of mental health conditions, how to start a supportive conversation and when and how to signpost a person to seek appropriate professional help. Learners will know how to recognise and manage stress. Learners will not diagnose or treat mental health conditions as this can only be carried out by healthcare professionals but will gain the knowledge to identify when a person may have a condition and know where they can go to get help. Suitability - Who should attend? Here are some examples of who may benefit from attending the RQF Level 1 Award in Awareness of First Aid for Mental Health: Employees and workers: This course is relevant for individuals in any industry who may encounter colleagues or clients experiencing mental health difficulties. It can be particularly valuable for human resources personnel, line managers, supervisors, or team leaders responsible for the well-being of employees. Teachers and educators: Professionals working in schools, colleges, or other educational institutions can benefit from this training to better understand and support the mental health needs of students. Healthcare and social care workers: Individuals working in healthcare or social care settings, such as nurses, care assistants, support workers, or counsellors, can enhance their understanding of mental health issues and improve their ability to provide appropriate support. Community and voluntary workers: People involved in community or voluntary organizations, including youth workers, social workers, volunteers, or community leaders, can gain valuable insights into mental health awareness and support. Personal relationships and caregivers: The Level 1 training can also be beneficial for individuals who have personal relationships with someone experiencing mental health challenges. This may include family members, friends, or caregivers who want to enhance their understanding and offer appropriate assistance. It is important to note that the Level 1 Award in Awareness of First Aid for Mental Health RQF is an introductory course and does not qualify participants to provide formal mental health interventions or therapy. However, it serves as a foundation for further training and can contribute to creating a more mentally healthy and supportive environment in various settings. Outcome / Qualification etc. Upon successful completion of the RQF Level 1 Awareness of First Aid for Mental Health course, participants can expect to achieve the following outcomes: Increased Awareness and Understanding: Participants will develop a basic awareness and understanding of mental health and mental health issues. They will gain knowledge about common mental health conditions, their signs and symptoms, and the importance of mental health in overall well-being. Recognition of Mental Health Signs: Participants will learn to recognize common signs of mental health issues in themselves and others. They will gain an understanding of the importance of early identification and intervention in promoting mental health and seeking appropriate support. Reduced Stigma and Improved Attitudes: The course aims to challenge stigmas and stereotypes associated with mental health. Participants will develop a more empathetic and supportive attitude towards individuals experiencing mental health challenges, promoting a positive and inclusive environment. Enhanced Communication Skills: Participants will learn basic communication skills for engaging with individuals experiencing mental health issues. They will gain an understanding of the importance of active listening, empathy, and non-judgmental communication in providing initial support. Signposting and Seeking Help: Participants will be equipped with knowledge about available resources, services, and support networks for mental health. They will learn about signposting individuals to appropriate professional help and self-help resources. Self-Care and Well-being Strategies: The course may provide participants with practical strategies for maintaining their own mental well-being. They may learn basic self-care techniques and stress management strategies to support their own mental health. Certificate of Completion: Upon successful completion of the course, participants will receive a certificate indicating their achievement of the RQF Level 1 Awareness of First Aid for Mental Health qualification. It's important to note that the Level 1 course provides a basic introduction to mental health awareness and first aid. It is not intended to provide participants with the qualifications or skills to diagnose or treat mental health conditions. Instead, it aims to promote mental health literacy, reduce stigma, and provide individuals with the knowledge to offer initial support and signposting to individuals in need. The Level 1 course can serve as a foundation for further learning and progression in the field of mental health. Individuals may choose to pursue higher-level courses or qualifications to develop more advanced skills and knowledge in mental health first aid or other related areas. Training Course Content MODULE 1 INTRODUCTION Session content Trainer/assessor introduction Learner introductions Course information • Administration • Learning outcomes and assessment criteria • Reasonable adjustments • Certification • Complaints and appeals • Assessment information Session duration 15 minutes MODULE 2 WHAT IS FIRST AID FOR MENTAL HEALTH? Session content Definitions Role and responsibilities of a First Aider The impact of mental health issues Mental health stigma Statistics Session duration 25 minutes MODULE 3 IDENTIFYING MENTAL HEALTH CONDITIONS Session content Mental health continuum Mental health risk factors Early warning signs Session duration 25 minutes MODULE 4 PROVIDING ADVICE AND STARTING A CONVERSATION Session content How to start a difficult conversation Non-judgemental listening skills When to contact the emergency services The First Aider’s own health and emotions Session duration 40 minutes MODULE 5 STRESS Session content What is stress? Causes of stress Effects of stress Coping strategies Session duration 25 minutes MODULE 6 MENTAL HEALTH CONDITIONS Session content Depression Anxiety Psychosis Eating disorders Suicide Self-harm Session duration 70 minutes MODULE 7 ASSESSMENT AND COURSE CLOSURE Session content Written assessment paper Course administration Course closure Session duration 40 minute Course delivery details Classroom-Based A minimum of 4 hours spread over at least one day. Ideally, the course should be run in one day, but must be completed within 2 weeks of starting the course, with each training session a minimum of two hours. Online/Virtual classroom The qualification has 2 assigned guided learning hours (GLH) and 5 hours total qualification time (TQT). GLH indicates the number of contact hours that the learner will have with the trainer/assessor. TQT includes GLH but considers unsupervised learning and is an estimate of how long the average learner will take to achieve the qualification. Why choose Madeleys First Aid Plus Founded in 2021 after Louise left 30 years in the NHS as an Advanced practitioner in A&E/ITU, had spent 1.5 years in Covid ITU Won FSB Best start-up business in the West Midlands in May 2023 Now trained 100's of delegates in Physical and Mental Health First Aid Expenses Travel costs and lunch required, there are many cafes and sandwich bars here in Much Wenlock to buy your lunch, you may eat it in the training room. All training material, books, qualification certificates are included in the price. Continuing Studies After completing the RQF Level 1 Awareness of First Aid for Mental Health course, individuals can consider various progression options to further their knowledge and skills in mental health support. Here are some potential pathways: RQF Level 2 Award in First Aid for Mental Health: This qualification builds upon the knowledge gained in the Level 1 course and provides a more comprehensive understanding of mental health issues and how to provide appropriate support. It covers topics such as recognizing mental health conditions, promoting well-being, and providing initial support to those in crisis. RQF Level 3 Award in Supervising First Aid for Mental Health: For individuals who aspire to take on leadership or supervisory roles in mental health support, the Level 3 qualification is a logical progression. It provides in-depth knowledge and skills to supervise and manage a team of individuals providing first aid for mental health. Continued Professional Development (CPD): Engaging in ongoing CPD activities is essential for staying updated with the latest developments in mental health support. Individuals can attend workshops, seminars, or conferences related to mental health, trauma, or specific areas of interest within the field. Applied Practice: Applying the knowledge gained from the Level 1 course in real-world settings is crucial for developing practical skills. Individuals can seek opportunities to work or volunteer in environments where mental health support is needed, such as community organizations, schools, or helplines. Mental Health Support Training Programs: There are various specialized training programs available that focus on specific aspects of mental health support, such as suicide prevention, trauma-informed care, or supporting individuals with specific mental health conditions. These programs can provide individuals with additional expertise and deepen their understanding of specific areas within mental health support. Higher Education: Individuals who wish to pursue a more in-depth study of mental health can consider higher education programs in psychology, counseling, social work, or related fields. These programs provide comprehensive knowledge and training in mental health support and may lead to professional certifications or degrees. It's important for individuals to research and explore progression options that align with their specific career goals, interests, and local requirements. Different countries or regions may have varying certification or training requirements for mental health support roles, so it's advisable to check with relevant regulatory bodies or professional associations for specific guidance.

Make Clothes
By The Stitchery Frome
Learn to make your own clothes using a paper pattern, in Frome Somerset

Search By Location
- CLO Courses in London
- CLO Courses in Birmingham
- CLO Courses in Glasgow
- CLO Courses in Liverpool
- CLO Courses in Bristol
- CLO Courses in Manchester
- CLO Courses in Sheffield
- CLO Courses in Leeds
- CLO Courses in Edinburgh
- CLO Courses in Leicester
- CLO Courses in Coventry
- CLO Courses in Bradford
- CLO Courses in Cardiff
- CLO Courses in Belfast
- CLO Courses in Nottingham