- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
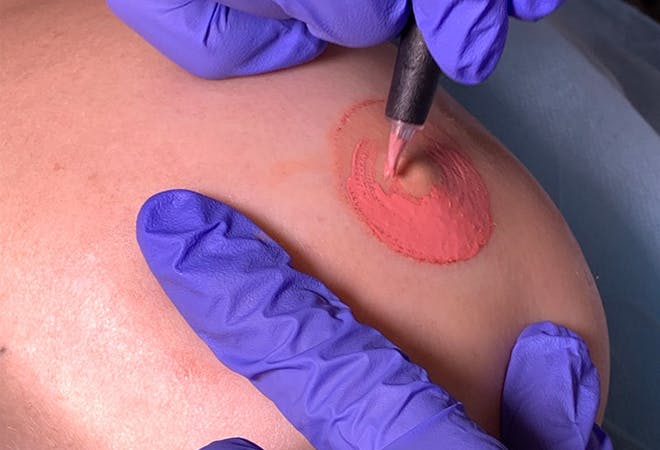
Areola Micropigmentation Training Course
By Cosmetic College
You will also learn how to visually improve scarring and areola asymmetries resulting from breast reduction and uplift surgical procedures. The specialist knowledge and techniques are gained through theory and step-by-step practice, which progresses from drawing on paper, to breast mound exercises and finally working on models. Course Prerequisites This course is suitable for those with no prior experience and is designed to provide the student with the ability to seek employment or start their own business upon qualification. Course Pre Study Before beginning practical training, students are required to complete: 30 hours of easy to access e-learning A series of online assessments Course Structure This is a 1 day course with intensive theory and practical sessions. All courses are kept intimate with a maximum of 4 students per course. Areas of study: The fundamental theory of cosmetic tattooing Anatomy and Physiology Health and Safety Colour Theory Client Assessment and Suitability Legal requirements, obtaining consent with consultation techniques and documentation Clinical setup procedures Pre-treatment drawing techniques Practical technique sessions on practice materials and model clients. Professional live demonstrations Industry leading pre and post treatment care for your clients Practical areas included in depth within the course are: Correct areola design planning Blending techniques Sanitation and sterilisation Correct station set up Skin colour matching analysis Needle specification
Foundation Lip Augmentation Course
By Cosmetic College
Our foundation lip filler course is designed for learners wanting to enter the industry at a beginner level and specialise in lip augmentation. Our goal at the Cosmetic College is to deliver the highest quality training in dermal fillers using the safest and most up-to-date techniques. Our courses are carried out in small groups; This enables us to provide you with a training programme tailored to your needs, with more time and emphasis on the hands-on practical sessions. Course Prerequisites This course is suitable for those with or without a medical background. It is designed to provide the student with the ability to seek employment or start their own business upon qualification. At a minimum, students will be required to be qualified at least one of the following: Medically qualified as a nurse, doctor or dentist with current registration with the NMC, GMC or GDC. NVQ Level 3 in Beauty Therapy, ITEC or HND 12 months of needling experience 6 Months micropigmentation experience and Anatomy & Physiology Level 3 Please note that if your qualification does not appear above, we offer a fast track access course for those completely new to the industry. Course Pre Study 40 hours of E-learning A series of online assessments 2 Days of Practical training Anatomy and physiology of the face Infection control Sharps and hazardous waste training First aid and anaphylaxis training Introduction to dermal filler injection techniques Elective and emergency dissolving Injection techniques practice Danger zones training Vessels, muscles, fat pads workshop Professional live demonstrations Legal requirements, obtaining consent with consultation techniques and documentation Practical training Clinical setup procedures Areas CoveredLips Professional KitDermal filler (Used for training) Numbing creamMassage gelChlorhexidine pre-injection wipesSterile treatment packsGlovesSurgical face masksSkin marker pencilsIce packConsultation forms

Myomectomy Course (Laparoscopic and Robotic)
By CCMIG
Total Laparoscopic Hysterectomy (TLH) Course in London for Gynaecology doctors, trainees and consultants. Theory and hands on training.

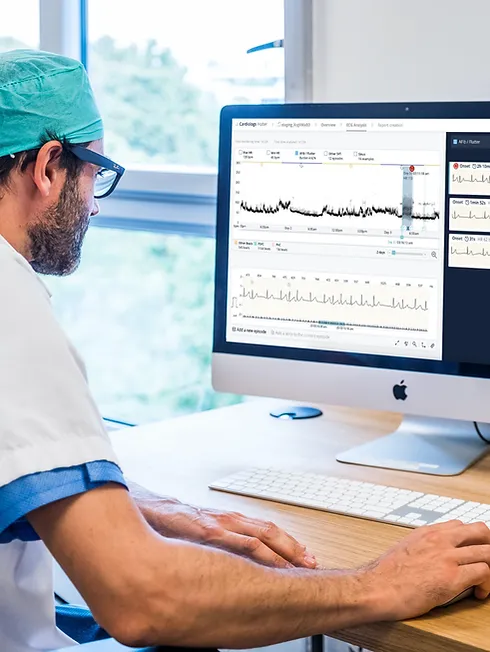
Learn how to perform and read an ECG ... Nationally Recognised Qualification OCN Accredited - Level 3 (advanced level) CPD Accredited - The CPD Certification Service Introduces you to the fundamentals of setting up and operating an ECG machine Includes patient preparation Produce a valid (error free) ECG Learn and understand ECG traces Recognise recordings that require urgent attention Basic understanding of English language required OPEN TO ALL APPLICANTS VIRTUAL CLASSROOM OPTION INCLUDES COMPREHENSIVE PRACTISE@HOME ECG TRAINING KIT Final interpretation of all ECG recordings is the responsibility of a medical professional.
A System Approach to Good Pharmacovigilance Practice
By Research Quality Association
Course Information In today's evolving landscape, pharmacovigilance (PV) systems face ongoing challenges due to global, national, and company-specific events. This course focuses on developing personnel equipped to navigate these complexities and improve the safe use of medicinal products. We emphasise continuous global thinking, communication, and strategic planning, ensuring adaptability across various levels. The course explores maintaining PV system functionality while adhering to regulatory requirements. Participants will apply European regulatory standards to enhance and sustain PV system effectiveness, contributing to improvement initiatives and ensuring operational integrity. The course covers PV system intricacies, regulatory compliance, and a 'systems approach' for auditors, quality assurance personnel, and PV practitioners. Through presentations and workshops, attendees gain insights into implementing and maintaining an effective PV system. Who should attend? Auditors Pharmacovigilance Quality System Managers Pharmacovigilance scientists The QPPV. Course benefits Throughout the course delegates will explore application of the legal requirements for the PV system and quality system and how to assure these systems. They will demonstrate their ability to contribute to: A systematic investigation of the pharmacovigilance system and its quality system Examination of how the pharmacovigilance system and quality system interact to achieve compliance. The risk-based approach to auditing the PV system and quality system The maintenance of 'inspection readiness' Explore how to investigate the complex PV system Discussions about how to monitor and maintain the PV system and assure compliance. Course Objectives Clarify what has to be done: Explore application of the legal requirements. Explore how to do what has to be done: Adopt a systemic approach to systematically investigate or implement and maintain the PV system and quality system Examine how a compliant PV system and a compliant quality system interact to achieve compliance with regulatory requirements for PV Explore how to investigate the complexity of the PV system. Discus how to identify what is missing or what needs to be improved: Discuss how to monitor and maintain the PV system and assure compliance. This course will assist delegates with: An understanding of key system principles, A practical approach to implementing, maintaining and monitoring the PV system and its quality system A procedure to share expertise to increase efficiency and confidence. This course is structured to encourage delegates to: Discuss and develop ideas - Share knowledge and experiences - Solve specific problems. By the end of the course delegates will be able to: Understand better the pharmacovigilance system, its quality system and how the components interact to achieve the objectives of pharmacovigilance Investigate, and analyse the pharmacovigilance system and to identify what is missing and what needs to be improved. Tutors Tutors will be comprised of (click the photos for biographies): Jana Hyankova Head of PV Department, IVIGEE Services a.s. Programme Please note timings may be subject to alteration. Day 1 08:30 Welcome, registration, course objectives and introduction to work groups Housekeeping notices, meet other delegates, explore how to work in your work group, course objectives. Clarify the definition and objectives of Pharmacovigilance. 09:30 The Regulatory Framework for Pharmacovigilance Identify the relevant regulations and directives. Explore GVP guidance, structure of the modules and standard format of each module. 10:00 The Pharmacovigilance System Exploration of how to organise what has to be done, communications. Exploration of the structures and processes for pharmacovigilance. 10:30 Break 11:00 Workshop 1 and Feedback Exploring an organisational model of the pharmaceutical company- cooperation between PV and other stakeholders. 12:00 The Quality System for pharmacovigilance Exploration of the structures, processes for the PV quality system and discussion of how it interacts with the pharmacovigilance system to meet the objectives of pharmacovigilance effectively and efficiently. 13:00 Lunch 14:00 Workshop 2 and Feedback The quality system puzzle. Explore the organisation of the PV quality system and how it interacts with the PV system. 14:30 The Quality System for pharmacovigilance Exploration of the structures processes for the PV quality system and discussion of how it interacts with the pharmacovigilance system to meet the objectives of pharmacovigilance effectively and efficiently. 15:00 Description of PV System 15:30 Break 15:30 Workshop 3 and Feedback The quality system puzzle Explore the organisation of the PV quality system and how it interacts with the PV system. 16:00 The Pharmacovigilance Safety Master File Construction of the Pharmacovigilance System Master File and its purpose. 17:00 Workshop 3 and Feedback Description of PV System. 18:00 End of Day Day 2 08:30 Drug Safety in the Clinical Trial Environment - Part 1 Information flow and responsibilities of the sponsor. 09:30 Workshop 4 and Feedback Drug Safety in the Clinical Trial environment: Information flow and responsibilities of the sponsor. 10:30 Break 11:00 Drug Safety in the Clinical Trial Environment - Part 2 Information flow and responsibilities of the sponsor. 12:00 Lunch 13:00 Workshop 5 and Feedback Drug Safety in the clinical trial environment: Information flow and responsibilities of the sponsor. 13:30 Processing of Safety Data Exploration of safety data processing, verification, validation, follow up, formatting and collation, reporting requirements, quality and data management. 15:00 Break 15:30 EudraVigilance Exploration of how EudraVigilance supports the PV system. 16:15 Signal Detection and Evaluation/Risk Benefit Assessment: Pharmacovigilance Risk Assessment Committee (PRAC): What is a signal? What are the regulatory requirements? How is signal detection and evaluation conducted? Qualitative and quantitative methods of signal detection. Risk benefit assessment. 17:00 Risk Management Plans A cornerstone of Pharmacovigilance safety communications, direct healthcare professional communication 18:00 End of Day Day 3 08:30 The Pharmacovigilance Risk Assessment Committee (PRAC) Exploration of how good practice is achieved. Composition, role and responsibilities. Examples of referrals. 09:15 Development Safety Update Reports (DSURs): Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 10:00 Periodic Safety Update Reports (PSURs)/Periodic Benefit Risk Evaluation Reports (PBRERs) Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 10:30 Break 11:00 Periodic Safety Update Reports (PSURs)/Periodic Benefit Risk Evaluation Reports (PBRERs) Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 12:00 Workshop 6 and Feedback To explore the compilation and submission of the PSUR. 13:00 Lunch 13:30 Role of the QPPV Exploration of the legal responsibilities of the QPPV and the MAH. 14:30 Break 15:00 Workshop 7 and Feedback To explore the challenges faced by the QPPV. 15:30 End of course Extra Information Face-to-Face Course Course material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device< Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking You will need a stable internet connection, a microphone and a webcam. CPD Points 23 Points Development Level Develop

Promoting Best Practice in Asthma and Anaphylaxis Training (Train the Trainer)
By Guardian Angels Training
Gain comprehensive knowledge and practical skills in asthma and anaphylaxis management with our evidence-based training course. Ideal for healthcare professionals, educators, and patient care providers. Optimise patient outcomes with effective emergency response protocols, prevention strategies, and patient education.
Search By Location
- Clinical Courses in London
- Clinical Courses in Birmingham
- Clinical Courses in Glasgow
- Clinical Courses in Liverpool
- Clinical Courses in Bristol
- Clinical Courses in Manchester
- Clinical Courses in Sheffield
- Clinical Courses in Leeds
- Clinical Courses in Edinburgh
- Clinical Courses in Leicester
- Clinical Courses in Coventry
- Clinical Courses in Bradford
- Clinical Courses in Cardiff
- Clinical Courses in Belfast
- Clinical Courses in Nottingham
