- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
613 Care courses in Leatherhead
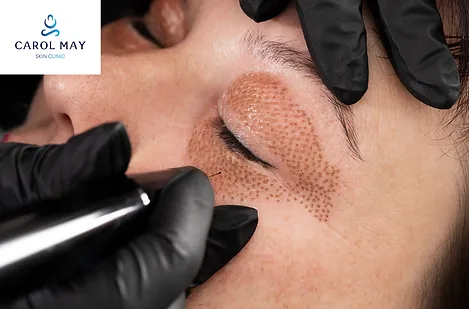
Plasma Fibroblast Training
By LMA Skin Clinic
Plasma pen fibroblast training for the face and body including soft surgery. Train one to one or very small groups. International accreditation. Lifelong support. Train with Carol May LMA Skin Clinic. On completion you will be able to offer your clients: Wrinkle rejuvenation & face lifting Hooded eyes & eye bags Glabella lines Nasal labia Marionette lines Neck lines Stretch marks & scar repair Mole removal Skin tags Cherry angioma Thread veins Xanthelasma Seborrheic keratosis plus many more soft surgery options You will benefit from online pre study plus in-clinic training and ongoing support Models can be provided if required You will receive a genuine medical grade plasma device (not electrical arcing) Total price is £2400 Your training fee can be paid in installments with Klarna We welcome national and International students
Green Hydrogen Production from Offshore Wind
By EnergyEdge - Training for a Sustainable Energy Future
About this Virtual Instructor Led Training (VILT) Hydrogen will play an increasingly critical role in the future of energy system as it moves forward to supplement and potentially replace fossil fuels in the long run. Offshore wind offers a clean and sustainable renewable resource for green hydrogen production. However, it can also be volatile and presents inherent risks that need to be managed. Even though offshore production of hydrogen has yet to achieve a high state of maturity, many current projects are already dealing with the conditions and effects of offshore production of hydrogen and are grappling with the technological requirements and necessary gas transportation with grid integration. This 2 half-day Virtual Instructor Lead Training (VILT) course will examine the technological options for on-site production of hydrogen by electrolysis (onshore or offshore directly at the platform) as well as the transport of hydrogen (pipeline or ship). This VILT course will also explore the economic considerations and the outlook on future market opportunities. There will be exercises for the participants to work on over the two half-days. This course is delivered in partnership with Fraunhofer IEE. Training Objectives By the end of this VILT course, participants will be able to: Understand the technological attributes and options for green hydrogen production based on electricity from offshore wind. Explore the associated economic analysis for offshore wind hydrogen production, including CAPEX, OPEX, LCOE and LCOH Identify the critical infrastructure and technical configuration required for offshore green hydrogen including transportation networks and grid connectivity Learn from recent findings from current Research & Development projects concerning the differences between onshore and offshore hydrogen production. Target Audience This VILT course is intended: Renewable energy developers and operators Offshore oil & gas operators Energy transport and marine operators Energy policy makers and regulators IPPs and power utilities Training Methods The VILT course will be delivered online in 2 half-day sessions comprising 4 hours per day, including time for lectures, discussion, quizzes and short classroom exercises. Course Duration: 2 half-day sessions, 4 hours per session (8 hours in total). Trainer Trainer 1: Your expert course leader is Director of Energy Process Technology Division at the Fraunhofer Institute for Energy Economics and Energy System Technology, IEE. The research activities of the division link the areas of energy conversion processes and control engineering. The application fields covered are renewable energy technologies, energy storage systems and power to gas with a strong focus on green hydrogen. From 2006 - 2007, he worked as a research analyst of the German Advisory Council on Global Change, WBGU, Berlin. He has extensive training experience from Bachelor and Master courses at different universities as well as in the context of international training activities - recently on hydrogen and PtX for partners in the MENA region and South America. He holds a University degree (Diploma) in Physics, University of Karlsruhe (KIT). Trainer 2: Your expert course leader is Deputy Head of Energy Storage Department at Fraunhofer IEE. Prior to this, he was the director of the Grid Integration Department at SMA Solar Technology AG, one of the world's largest manufacturers of PV power converters. Before joining SMA, he was manager of the Front Office System Planning at Amprion GmbH (formerly RWE TSO), one of the four German transmission system operators. He holds a Degree of Electrical Engineering from the University of Kassel, Germany. In 2003, he finished his Ph.D. (Dr.-Ing.) on the topic of wind power forecasting at the Institute of Solar Energy Supply Technology (now known as Fraunhofer IEE) in Kassel. In 2004, he started his career at RWE TSO with a main focus on wind power integration and congestion management. He is Chairman of the IEC SC 8A 'Grid Integration of Large-capacity Renewable Energy (RE) Generation' and has published several papers about grid integration of renewable energy source and forecasting systems on books, magazines, international conferences and workshops. Trainer 3: Your expert course leader is Deputy Director of the Energy Process Technology division and Head of the Renewable Gases and Bio Energy Department at Fraunhofer IEE. His work is mainly focused on the integration of renewable gases and bioenergy systems into the energy supply structures. He has been working in this field since more than 20 years. He is a university lecturer in national and international master courses. He is member of the scientific advisory council of the European Biogas Association, member of the steering committee of the Association for Technology and Structures in Agriculture, member of the International Advisory Committee (ISAC) of the European Biomass Conference and member of the scientific committees of national bioenergy conferences. He studied mechanical engineering at the University of Darmstadt, Germany. He received his Doctoral degree on the topic of aerothermodynamics of gas turbine combustion chambers. He started his career in renewable energies in 2001, with the topic of biogas fired micro gas turbines. Trainer 4: Your expert course leader has an M. Sc. and she joined Fraunhofer IEE in 2018. In the Division of Energy Process Technology, she is currently working as a Research Associate on various projects related to techno-economic analysis of international PtX projects and advises KfW Development Bank on PtX projects in North Africa. Her focus is on the calculation of electricity, hydrogen and derivative production costs (LCOE, LCOH, LCOA, etc) based on various methods of dynamic investment costing. She also supervises the development of models that simulate different PtX plant configurations to analyze the influence of different parameters on the cost of the final product, and to find the configuration that gives the lowest production cost. She received her Bachelor's degree in Industrial Engineering at the HAWK in Göttingen and her Master's degree in renewable energy and energy efficiency at the University of Kassel. POST TRAINING COACHING SUPPORT (OPTIONAL) To further optimise your learning experience from our courses, we also offer individualized 'One to One' coaching support for 2 hours post training. We can help improve your competence in your chosen area of interest, based on your learning needs and available hours. This is a great opportunity to improve your capability and confidence in a particular area of expertise. It will be delivered over a secure video conference call by one of our senior trainers. They will work with you to create a tailor-made coaching program that will help you achieve your goals faster. Request for further information about post training coaching support and fees applicable for this. Accreditions And Affliations

People Handling Instructor and Assessor
By Guardian Angels Training
Gain certification as a safe people handling instructor and assessor with our comprehensive course. Equip yourself with the necessary skills and knowledge to effectively train and assess others in safe manual handling techniques.

Foundations of Immunisation and Vaccines for Non-Registered Practitioners
By Guardian Angels Training
The "Foundations of Immunisation and Vaccines for Non-Registered Practitioners" course is fully compliant with the National Minimum Standards and Core Curriculum for Immunisation Training and is designed to equip non-registered healthcare professionals with a solid understanding of immunisation concepts, vaccine administration, and the importance of vaccination in public health.
Primary Angioplasty & Cardiac Emergencies
By M&K Update Ltd
Awareness of what is happening during the rupture of a coronary plaque assists the attendee in understanding why such a microscopic event can have such a catastrophic outcome.

Exercise Tolerance Testing
By M&K Update Ltd
Aimed at cardiac professionals to develop their Exercise Tolerance Testing (ETT) skills.

Chest Pain Assessment & Management
By M&K Update Ltd
An opportunity to learn about a structured assessment of chest pain. Primarily looking at Acute Coronary Syndrome (ACS), but also other differentials such as myopericarditis, dissection.

Arrhythmia Interpretation & Management
By M&K Update Ltd
An opportunity to learn about various heart rhythm disturbances, their aetiology, management and treatment strategies.

Good Clinical Practice Auditing - Principles and Practice
By Research Quality Association
Course Information Our comprehensive course is used as a gateway to those stepping into the world of auditing clinical studies. Tailored for those already acquainted with Good Clinical Practice (GCP) and those transitioning from other audit disciplines, this programme stands as a pivotal guide. Pre-existing knowledge of GCP will significantly enhance your learning experience in auditing against these guidelines. How is this course run? Engage in immersive workshops providing hands-on practice with auditing techniques in a GCP context. Our seasoned tutors, boasting extensive audit experience, intertwine theory with practical insights drawn from their own professional journeys. What will I learn? A comprehensive understanding of the historical backdrop and objectives driving Good Clinical Practice, incorporating the latest industry developments Solid grounding in quality assurance activities aligned with regulatory standards Insight into potential pitfalls within clinical trials and the pivotal role of auditors in addressing these issues Clarity on the roles and responsibilities inherent to clinical trials auditing Exposure to a diverse range of audit techniques complemented by illustrative examples and supportive documents A nuanced understanding of regulatory inspectors' activities Expanded professional networks to propel your auditing career forward. Benefits include: A clear understanding of the role of the auditor under Good Clinical Practice improved audits Improved Good Clinical Practice compliance for your clinical trials. This course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Examine particular aspects of Good Clinical Practice. Tutors Tutors will be comprised of (click the photos for biographies): Rosemarie Corrigan EVP Global Quality, Worldwide Clinical Trials Cathy Dove Director and Owner, Dove Quality Solutions Julie Kelly Associate Director, Clinical Quality Assurance, Corcept Therapeutics Susana Tavares Director of Research Quality Assurance, - Programme Please note timings may be subject to alteration. Day 1 12:30 Registration 13:00 Welcome and Objectives for the first day of the course 13:30 Laying the Foundations Introduction to the clinical development process, the concepts of quality assurance, quality control and audit. 14:30 Break 15:00 Patient Protection Requirements for informed consent and ethics committee. Access to source documentation. Including a patient protection exercise. 16:05 Workshop 1 - Case Study on Informed Consent 16:45 End of Day Questions and Answers 17:00 Close of Day Day 2 08:50 Questions and Answers from Day 1 09:00 Effective Site Audits The procedures involved in selecting and setting up audits at investigator sites. 09:40 Workshop 1 - Planning the Effective Audit 10:30 Break 10:45 Source Data Verification The need for and purpose of verifying data. 11:25 Workshop 2 - Source Data Verification 12:30 Lunch 13:30 IMP Management The requirements surrounding the distribution of investigational medicinal products. Accountability from release to destruction. 14:15 Critical Document Audits The conduct of other study specific audits including protocols, databases and reports. 15:00 Break 15:15 Non-compliance Determining the acceptability of data. 16:00 Fraud - Fact or Fiction? How to identify fraud and its consequences 16:45 End of Day Questions and Answers 17:00 Close of Day Day 3 08:50 Questions and Answers from Days 1 and 2 09:00 Auditing Third Parties A review of audits of contract research organisations. 10:00 System Audits The concept of auditing processes across many clinical trials, including a practical exercise in process mapping. 10:45 Break 11:00 Workshop 3 - Process Mapping 11:45 Effective Audits Where theory meets reality. 12:30 Lunch 13:20 Audit Reports - Closing the Loop An examination of the processes which follow the evidence gathering phase of the audit. 14:20 Workshop 4 - Audit Reports Audit reports, corrective and preventive action. 15:00 Break 15:10 Regulatory Inspection Auditors and regulatory inspections -how the QA team can help the organisation to perform during a regulatory inspection. 15:55 Final Questions and Answers 16:10 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 17 Points Development Level Develop

Certified Scrum Professional-ScrumMaster: In-House Training
By IIL Europe Ltd
Certified Scrum Professional®-ScrumMaster® (CSP®-SM): In-House Training Certified Scrum Professionals challenge their teams to improve the way Scrum and Agile principles are applied. They have demonstrated experience, documented training, and proven knowledge in Scrum. Are you ready to take your knowledge and skillset in your role as Scrum Master to the next level? If so, it's time to elevate your career further by earning the Certified Scrum Professional®-ScrumMaster (CSP®-SM) certification. What you will Learn Learn to find practical solutions and improve your implementation of Scrum in the workplace. Aside from the pride gained and earning potential of attaining CSP® level, you can also: Attend exclusive CSP® events with other leaders in Scrum and Agile Attract more recruiters and command a higher rate of pay Establish a gateway and milestone toward becoming CST®, CEC, or CTC Receive a free premium subscription to the world's largest Agile assessment and continuous improvement platform, Comparative Agility®

Search By Location
- Care Courses in London
- Care Courses in Birmingham
- Care Courses in Glasgow
- Care Courses in Liverpool
- Care Courses in Bristol
- Care Courses in Manchester
- Care Courses in Sheffield
- Care Courses in Leeds
- Care Courses in Edinburgh
- Care Courses in Leicester
- Care Courses in Coventry
- Care Courses in Bradford
- Care Courses in Cardiff
- Care Courses in Belfast
- Care Courses in Nottingham