- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
1480 Courses
The Emergency treatment of Adrenal Crisis
By Guardian Angels Training
Gain essential knowledge and skills to recognise, manage, and provide prompt emergency treatment for adrenal crisis with our course. Ideal for healthcare professionals.

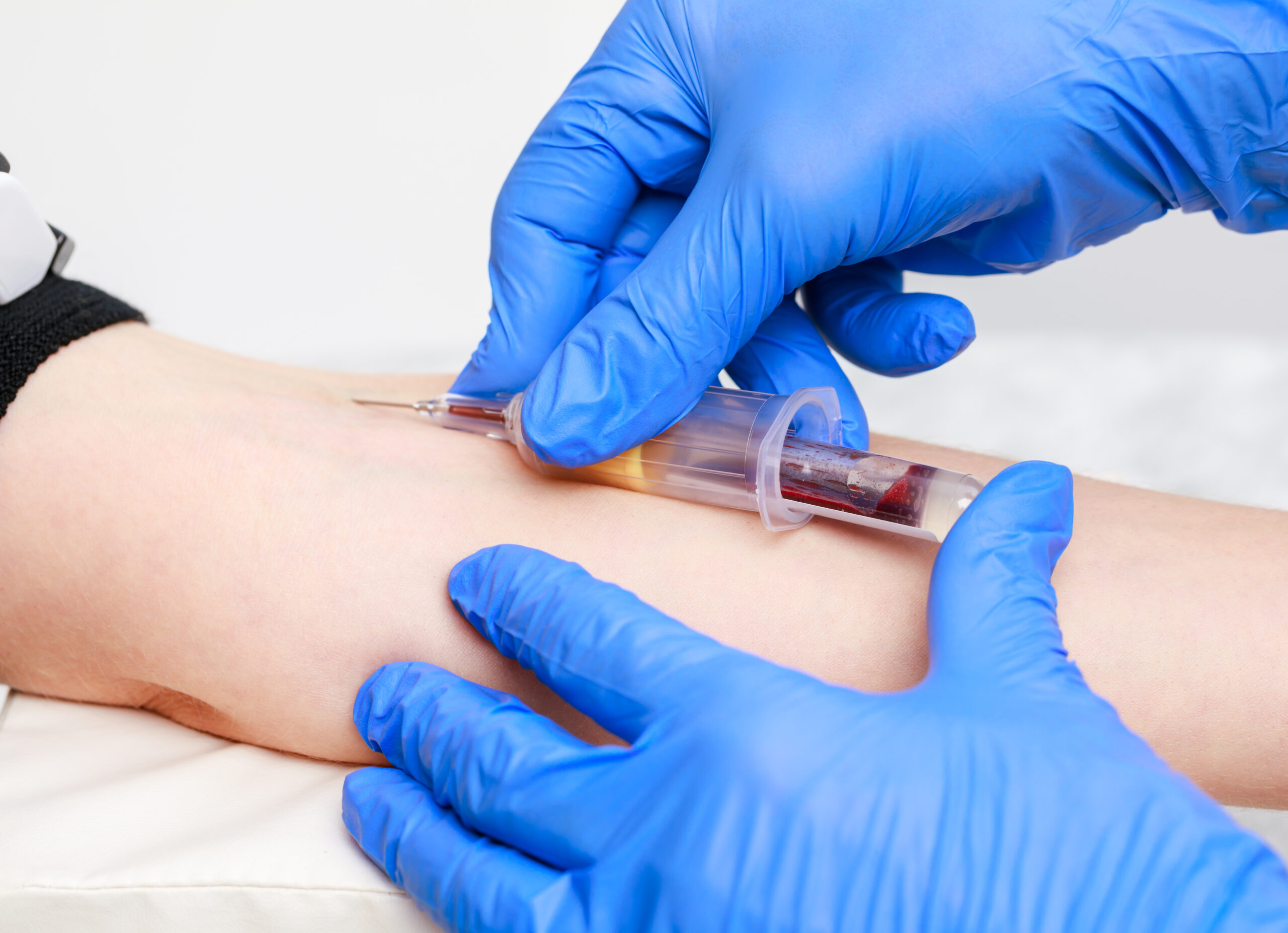
Promoting Best Practice in Venepuncture Instruction
By Guardian Angels Training
Enhance your venepuncture skills with our comprehensive course. Learn evidence-based techniques, infection prevention measures, patient-centered communication, and ethical considerations to ensure safe and effective practices.
People Handling Instructor and Assessor
By Guardian Angels Training
Gain certification as a safe people handling instructor and assessor with our comprehensive course. Equip yourself with the necessary skills and knowledge to effectively train and assess others in safe manual handling techniques.

Train the Trainer / Instructor Course in Nasogastric Tube Insertion and Feeding
By Guardian Angels Training
Gain comprehensive knowledge and practical skills for safe and effective nasogastric tube insertion and feeding techniques with our "Promoting Best Practice in Nasogastric Tube Insertion and Feeding Tuition" course. Optimise patient safety, comfort, and outcomes with evidence-based best practices.

Level 3 Endorsed Award in Delivering Health and Social Care Training (Healthcare Train the Trainer)
By Guardian Angels Training
Gain expertise in healthcare training with our Level 3 Endorsed Award in Delivering Health and Social Care Training. Our comprehensive program equips you with the skills and knowledge to become a proficient trainer in the healthcare sector.

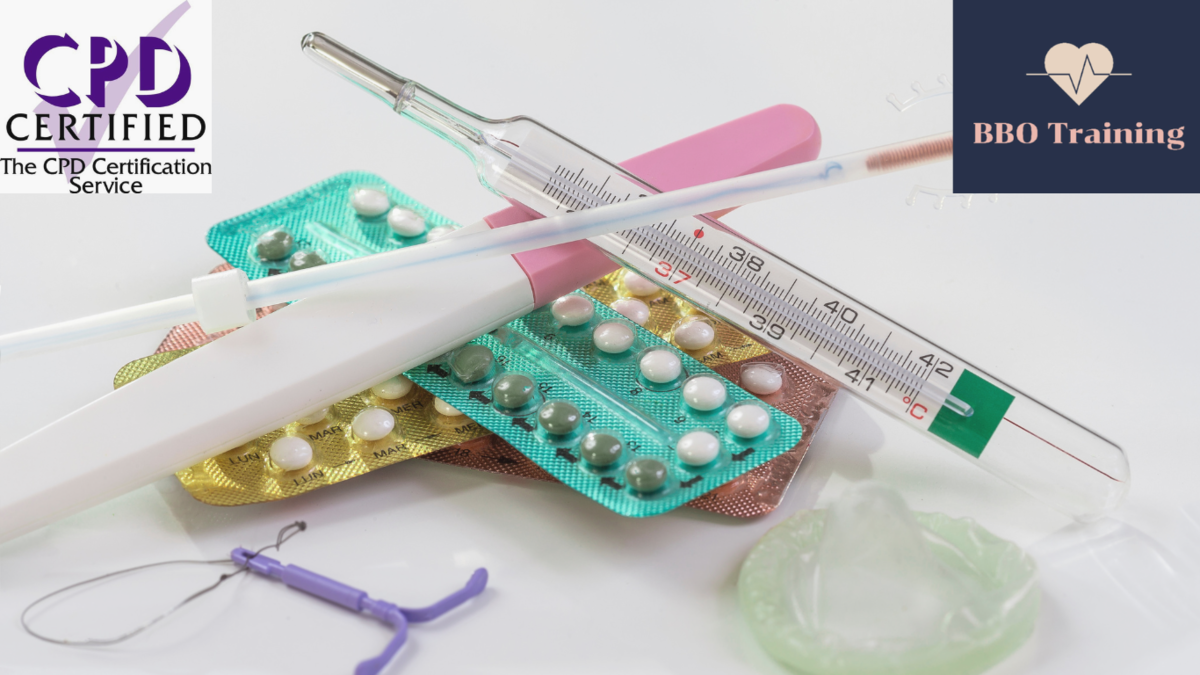
Introduction to Contraception for Healthcare Professionals (CPD Accredited)
By BBO Training
We are delighted to invite you to BBO Training's 'Accredited Introduction to Contraception for Healthcare Professionals' training, a comprehensive 2-day course designed to enhance your knowledge and skills in the field of contraception. During this training, we will cover all contraception methods, including long-acting reversible contraceptives (LARC's), emergency contraception, and basic asymptomatic sexually transmitted infection (STI) screening. This course is specifically tailored for Nurse Associates, Practice Nurses, Pharmacists, Paramedics, and other allied health professionals who are seeking to expand their understanding of basic contraceptive advice. By the end of the training, you can expect to achieve the following learning outcomes: 1. Gain a comprehensive understanding of various contraception methods, including their efficacy, benefits, and limitations. 2. Develop the ability to provide basic contraceptive advice and guidance to patients, taking into account their individual needs and preferences. 3. Acquire the skills necessary to perform simple pill checks and administer repeat Depo injections confidently. 4. Learn how to effectively discuss and recommend long-acting reversible contraceptives (LARC's) to patients, considering their suitability and effectiveness. 5. Understand the importance of asymptomatic STI screening and learn how to offer this service appropriately, following a period of supervised practice.
Search By Location
- Healthcare professions Courses in London
- Healthcare professions Courses in Birmingham
- Healthcare professions Courses in Glasgow
- Healthcare professions Courses in Liverpool
- Healthcare professions Courses in Bristol
- Healthcare professions Courses in Manchester
- Healthcare professions Courses in Sheffield
- Healthcare professions Courses in Leeds
- Healthcare professions Courses in Edinburgh
- Healthcare professions Courses in Leicester
- Healthcare professions Courses in Coventry
- Healthcare professions Courses in Bradford
- Healthcare professions Courses in Cardiff
- Healthcare professions Courses in Belfast
- Healthcare professions Courses in Nottingham
