- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
32726 Courses
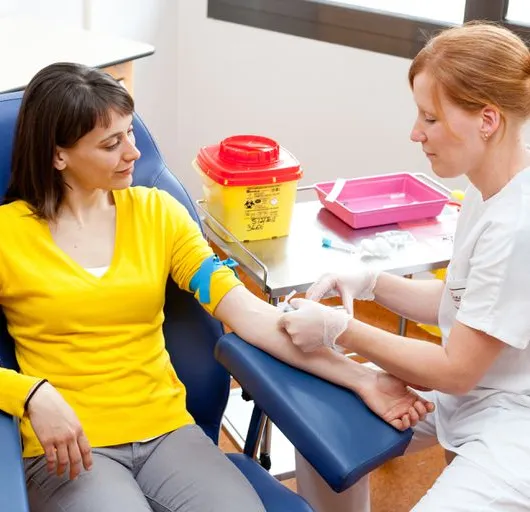
Introduction to Phlebotomy Course (GPT003) - 1 DAY Classroom - Part 1 online + Part 2 Classroom
4.6(39)By Geopace Training
Learn how to take blood ... train as a Phlebotomist Nationally Recognised Qualification No previous experience or qualifications needed OCN Accredited - Level 3 (advanced) CPD Accredited (The CPD Certification Service) Covers all steps up to live blood draw Practise on artificial arm and fake blood! Basic understanding of English language required OPEN TO ALL APPLICANTS
Tackle Stress Before It Tackles You! Work-related stress affects 875,000 people every year, and its impacts go beyond the workplace—affecting your mind, body, and personal life. But it doesn’t have to be this way. Join our Stress Management Workshops to: ✔️ Understand the difference between stress and pressure ✔️ Learn the causes of stress in and out of the workplace ✔️ Discover practical coping strategies and build mental resilience These workshops are packed with insights, tools, and strategies to help you take control of your stress levels and improve your well-being—personally and professionally. There are two different ones to choose from - a 2 hour workshop and a 4 hour workshop! Course Contents of 2 hour course: What is Stress Stress versus Pressure Statistics Absenteeism, Presenteeism and Leaveism Workplace Causes of Stress Personal Causes of Stress Short-Term and Long-Term Effects of Stress Coping Strategies Mental Resilience Benefits of this Workshop: In 2022/23. 875,000 people suffered from work-related stress, depression or anxiety The affects of stress are far reaching, affecting one's mind, body, social and personal life Become more aware of what stresses you, what is does to you and find ways to reduce those stress levels

CPD Accredited, Interactive Workshop 2 hr session Learn all about the Five Steps to Mental Wellbeing and how they could be applied into your life Learn from the comfort of your own home Course Contents: Introduction to the Five Steps to Mental Wellbeing The Science Behind the Five Steps Learn about the Five Steps themselves and how to apply them into our own life - Connect - Be Active - Keep learning - Give to others - Be mindful Benefits of this Short Course: People's wellbeing have been under great strain. Stressful lives lead to people suffering from mental health problems. The Five Steps to Mental Wellbeing are a scientifically proven method of improving one's mental wellbeing, and increasing one's general emotional resilience In this Five Steps to Mental Wellbeing workshop, we'll show you what you can do to improve your own mental wellbeing and resilience, and can help you help others do the same!

CPD Accredited, Interactive Short Course 2 - 4 hr sessions What is Attention Deficit Disorder? How can you best understand, and thus cope and help those affected? Course Contents: What is ADHD ADHD as part of the Autistic Spectrum How to help you cope if you have ADHD How to cope with children who have ADHD Great for teachers and TAs in schools, others who work with people who have ADHD, and of course, those with ADHD themselves Benefits of this Short Course: Boys are around 4 x as likely to have ADHD Worldwide, 5% of people have it - that is 1 out of every 20! The figure for this is estimated to be higher in the UK This means that every single classroom in the UK will have at least one child or young people with Attention Deficit Hyperactive Disorder Learn more about the condition and how you can help them cope and take part in 'normal' life.

CPD Accredited, Interactive Short Course 2 or 3.5 hour hr sessions What are the autistic spectrum disorder, autism and asperger's syndrome? How can you best understand, and thus help, those affected? Course Contents of the shorter course: What is ASD Autism and Asperger's Syndrome Understanding those on the Spectrum How to approach and help those on the Autistic Spectrum Great for teachers and TAs in schools, and for those working in care with older people with ASD Benefits of this Short Course: About 1% of the population is thought to be on the Autistic Spectrum That is several children per nursery and primary school and a significant amount of young people in secondary schools Many with Asperger's Syndrome will also go to university The way they experience the world is very different to 'neuro-typical' people Understanding their condition helps to support them and give them the best chance

Learners will be introduced to EAS as part of the fire safety solution for tall residential properties. This CPD course provides learners with an understanding of the requirements of BS 5839-1 in relation to, design, installation, commissioning, and maintenance of EAS.

18th edition courses in Kent
By MJ Electrical Training
City & Guilds 18th edition course with 2382-22 final exam only £264.00 Inc VAT. Exams available every week across the UK, same day results, quick certificate, best prices..

Awareness of First Aid for Mental Health
By Madeleys First Aid Plus
🧠 Mental Health Matters — In Every Workplace 💬 Did you know? 📊 1 in 4 people in the UK experience a mental health condition each year 💼 1 in 6 employees face common mental health challenges at work This 4-hour Mental Health Awareness qualification helps break the silence and stigma by equipping you to: ✅ Recognise signs of mental health conditions 🗣️ Start supportive conversations 📍 Signpost to professional help 🌿 Understand and manage workplace stress You won’t be diagnosing — but you will be making a difference. 📅 Join us in creating safer, more supportive workplaces. #MentalHealthAwareness #WorkplaceWellbeing #EndTheStigma #SupportEachOther #MentalHealthTraining #BeThere

Search By Location
- cpd Courses in London
- cpd Courses in Birmingham
- cpd Courses in Glasgow
- cpd Courses in Liverpool
- cpd Courses in Bristol
- cpd Courses in Manchester
- cpd Courses in Sheffield
- cpd Courses in Leeds
- cpd Courses in Edinburgh
- cpd Courses in Leicester
- cpd Courses in Coventry
- cpd Courses in Bradford
- cpd Courses in Cardiff
- cpd Courses in Belfast
- cpd Courses in Nottingham

