- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
2101 Courses
ISO 7101 Healthcare Quality Management System Lead Auditor Course provides participants with the knowledge and skills necessary to lead audits of quality management systems within healthcare organizations. This comprehensive training program covers the principles, processes, and practices of auditing healthcare quality management systems, focusing on key standards and regulations applicable to the healthcare industry.

NPORS Report of Thorough Examination (N019)
By Dynamic Training and Assessments Ltd
NPORS Report of Thorough Examination (N019)

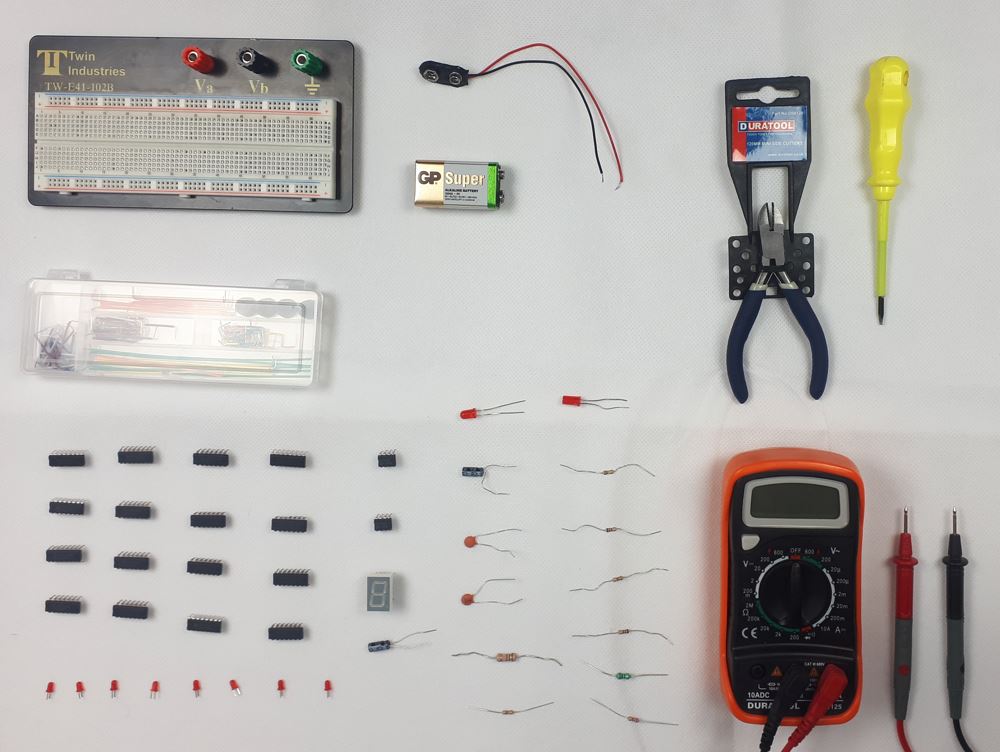
Digital Electronics Course Online
By Hi-Tech Training
This Digital Electronics Course is designed to give practical knowledge of the type of electronic circuitry used in a modern Computer System or in any type of Computer Controlled equipment such as Photocopiers, Cash Registers, Tablets, mobile phones and many other types of IT equipment. Digital Electronics involves the use of Silicon chips (Integrated Circuits). The internal structure of a computer is to a large extent comprised of Digital Electronic Circuits.
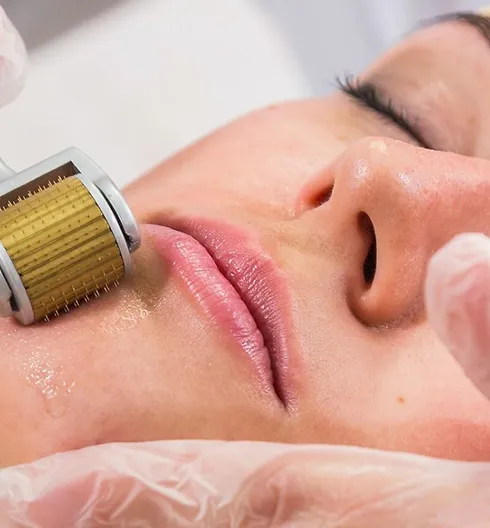
Platelet-rich Plasma (PRP) treatments Nationally Recognised Qualification No previous experience or qualifications needed Open College Network Accreditation Level 4 (as required for minimally invasive procedures) Covers standards set by HEE Employed (salon) or Self-Employed opportunities Basic understanding of English language required OPEN TO ALL APPLICANTS
A five day Refrigeration Electrics Maintenance course aimed at anyone involved with refrigeration, air conditioning and heat pump electrical control systems. The Refrigeration Electrics Maintenance course covers the three elements which are common to most refrigeration and air conditioning systems, namely, protection, control and motors. For example, a typical air conditioning split system will have protection provided by the fuse in the fused connection unit, and further protection provided to the cable feeding the fused connection unit, probably by a circuit breaker, control provided by thermostat and time clock etc… and motor driven fans and compressor. During the Refrigeration Electrics Maintenance course emphasis is also placed upon the applicable wiring regulations to ensure the constructed system is compliant with BS7671:2018 The aim of the Refrigeration Electrics Maintenance course is to provide enough knowledge to allow maintenance and fault finding to be safely and effectively carried out. The Refrigeration Electrics Maintenance course comprises of: Essential Electrics Module City and Guilds 2382-22 Level 3 – 18th Edition Wiring Regulations Refrigeration Electrics Module The Refrigeration Electrics Maintenance course costs include examination entry fees.

This module covers the requirements for TExT and how to carry out the Thorough Examination and Test process for on-tool dust extraction machines. To complete the training you will need a working knowledge of machinery and have operated or completed Combined Inspections and Tests (PAT) on machines.

This is a two week programme incorporating eight different modules including: Module 1 - Basic Electrics and PAT Module 2 - Engines / Power Systems Module 3 - Non-Mechanical Equipment Module 4 - Basic Hydraulics and Hydrostatic Drive Module 5 - Powered Access Equipment Module 5 LLA - Low Level Access specific element Module 6 - Workshop H&S Essentials Module 7 - Materials Hoists including Sumner 2000 series and Genie SLAs During the programme the learner is inducted on their Level 2 NVQ in Plant Maintenance or Level 3 in Thorough Examination. One week with us and then a period back at work (4 weeks or less) to gather work based evidence and then a further week back at our Penybont training centre At 1/2 a point per course the training is worth up to 4 points towards your IPAF CAP certification. The Level 2 PM is worth a further 2 points and the Level 3 Thorough Examination is worth 4 points.

Dustcontrol UK Ltd - Thorough Examination and Test (TExT) of Dust Extractors
By Learning for Hire Limited
This module covers the requirements for TExT and how to carry out the Thorough Examination and Test process for on-tool dust extraction machines. To complete the training you will need a working knowledge of machinery and have operated or completed Combined Inspections and Tests (PAT) on machines.

The Central Heating Controls Wiring and Fault Finding course is a two day-day short course aimed at anyone involved in the construction, commissioning, inspection & testing or maintenance of central heating electrical control systems. The Central Heating Controls Wiring and Fault Finding course covers all the commonly used control systems in use today and is focused on the ‘practical’ construction and commissioning along with the relevant fault finding techniques. The Central Heating Controls Wiring and Fault Finding course requires an understanding of electrical principles and cable termination skills. A requirement of this course is the successful completion of their Essential Electrics examination prior to sitting the central heating controls wiring & fault finding examinations. Please ring if clarification is needed on this point. There are two 20 minute open book examinations and the associated practical assessments. A third examination and associated assessment will be required for candidates not holding the Essential Electrics unit. The Central Heating Controls Wiring and Fault Finding course comprises of: Short theory sessions introducing the conventional wiring systems, ‘Y’ Plan, ‘S’ Plan, ‘C’ Plan, Etc. Detailed practical workshop sessions, undertaking the construction, testing and commissioning of systems Fault finding and maintenance tasks performed on working systems Examination and practical assessment preparation ready for the assessments Evaluation of the system control function (why and how it works) Each student will work on their own system, and will have plenty of time to absorb and understand how each systemworks. Advise will be given on suitable ‘tooling’ and test equipment. These are nationally recognizable qualifications which are fast becoming an essential requirement for this type of work. The course costs include comprehensive course notes and examination entry fees.

ProQual NVQ Level 3 Diploma - Testing, Inspecting and Thorough Examination of Plant, Machinery, Equipment or Accessories
By Learning for Hire Limited
ProQual NVQ Level 3 Diploma - Testing, Inspecting and Thorough Examination of Plant, Machinery, Equipment or Accessories - on-site assessment - we come to you

Search By Location
- Examination Courses in London
- Examination Courses in Birmingham
- Examination Courses in Glasgow
- Examination Courses in Liverpool
- Examination Courses in Bristol
- Examination Courses in Manchester
- Examination Courses in Sheffield
- Examination Courses in Leeds
- Examination Courses in Edinburgh
- Examination Courses in Leicester
- Examination Courses in Coventry
- Examination Courses in Bradford
- Examination Courses in Cardiff
- Examination Courses in Belfast
- Examination Courses in Nottingham