- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
41 Courses
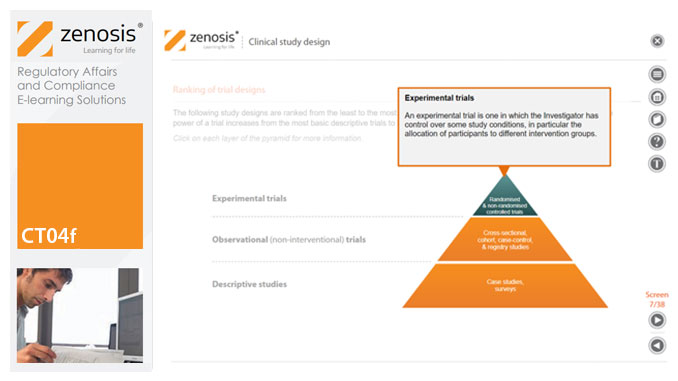
CT04f - Clinical study design
By Zenosis
Clinical trial design establishes the framework upon which the clinical trial process will be conducted, and sets the objectives of the trial. The application for marketing approval, submitted to the regulatory authorities, will provide clinical data reflecting the trial design. Since trial design impacts the whole drug development process and lifecycle, particular care and due diligence is essential. This short course provides an overview of the main types of study design.
CT09: Good Clinical Practice Inspections and Audits
By Zenosis
The module describes general principles of GCP inspection and audit, discusses preparation for an inspection, and sets out in detail what European and US FDA inspectors will examine. Finally it describes post-inspection actions by the regulator and the inspected party.

CT11: How to Gain Authorisation for Clinical Research Under the EU Clinical Trials Regulation
By Zenosis
This course sets out the procedures that sponsors need to follow to gain authorisation to conduct clinical trials under the Regulation, and it summarises and links to the extensive guidance available from the European Commission and the European Medicines Agency. Its companion course CT12 sets out the procedures that sponsors need to follow to conduct authorised clinical trials in compliance with the Regulation. The two courses therefore provide an ideal foundation for understanding and complying with the new law.
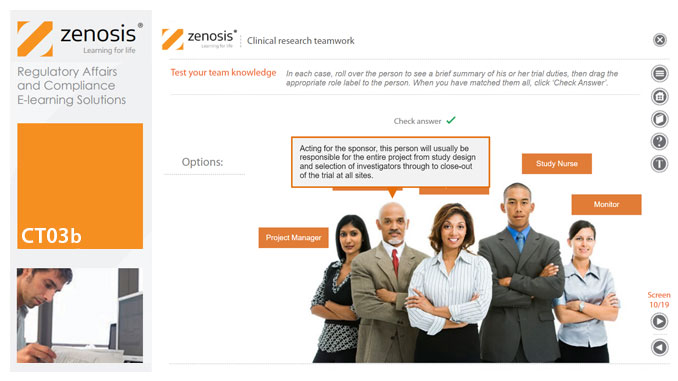
CT03b - Clinical research teamwork
By Zenosis
A clinical trial, particularly a late-phase commercial study, is a major project requiring collaboration between the sponsor and staff or contractor, on the one hand, and the clinical investigator(s) and other healthcare professionals on the other. Good communication among all parties is essential. In this short course we introduce the major roles in a typical clinical research project and outline their duties.
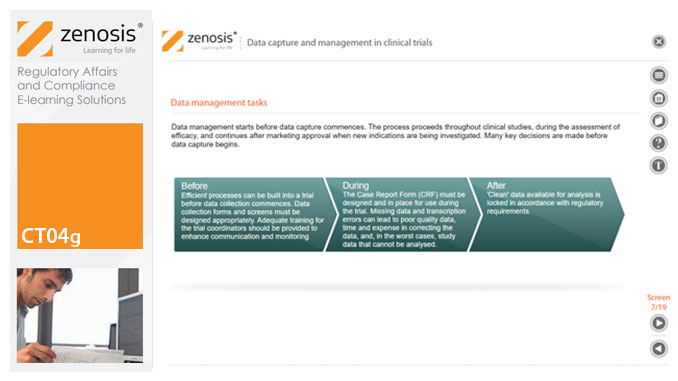
CT04g - Data capture and management in clinical trials
By Zenosis
Capture and management of clinical trial data is a challenge. The industry is under pressure to obtain and analyse such data more quickly, while maintaining data integrity, so that products can be brought to market sooner. Effective planning and adequate resources can ensure clinical trials yield high quality data within strict timelines and budget requirements, at the same time satisfying regulatory standards. This short course describes the purpose of data capture and explores efficiencies in data management as part of the evolving regulatory landscape.
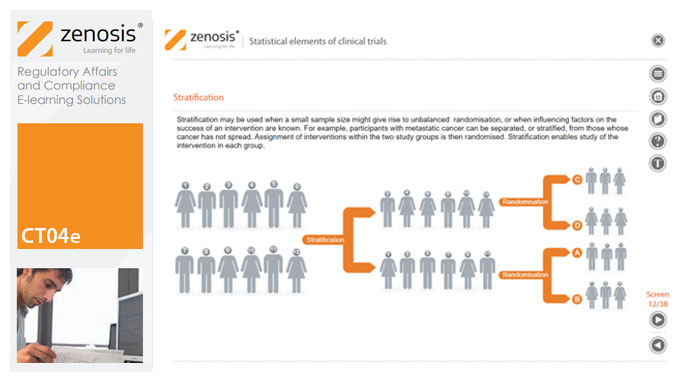
CT04e - Statistical elements of clinical trials
By Zenosis
Analytical statistical elements are essential concepts in the design of clinical trials. This analysis helps us to understand whether a conclusion from a study of a sample of the target population applies generally to that population as a whole. In particular, it helps us to answer the question: Did the treatment effect in the given study occur just by chance? The statistical elements of a well-controlled study minimise the chances of drawing the wrong conclusions, by providing clear thresholds for such errors. The basic statistical elements of a clinical trial include eligibility criteria, randomisation, sample size, power, and blinding, and these are discussed in this short course.
ICT03: Assuring Data Integrity in Clinical Research
By Zenosis
Pharmaceutical, biotechnology and medical device companies and clinical researchers need to assure regulatory authorities of the reliability of the data that they generate during product development and testing – that is, to demonstrate data integrity. Practices that provide assurance of data integrity in clinical research are required by law and/or established as expectations in regulatory guidance. The data are reviewed in regulatory applications or during regulatory inspections of clinical trial sponsor and investigational sites. Inadequacies of data integrity are frequently reported by inspectors and result in regulatory actions against the organizations or individuals concerned. This course explains the requirements and describes principles and practices that should be followed by trial sponsors, investigators and other clinical research personnel to assure regulators of data integrity.

In a world where medical advancements and ethical considerations intertwine, there exists a bundle that unveils the intricacies of this captivating field: the Medical Law And Ethics Diploma. Step into a realm where legal frameworks converge with the noble pursuit of providing quality healthcare. This bundle, carefully curated with a range of enlightening courses, offers a gateway to understanding the dynamic landscape of medical law and ethics in the UK. By immersing oneself in this Medical Law And Ethics bundle, you'll unlock the secrets of legislation pertaining to access to health, treatment, and medical reports. Secondly, the importance of patient welfare and ethics cannot be overstated. As a learner, you'll explore the intricacies of legislation on adult support, mental health, and abortion, equipping yourself with the knowledge to navigate ethically complex scenarios. Moreover, this Medical Law And Ethics bundle delves into crucial areas such as public health, coronavirus legislation, and the control and administration of medicines. The courses encompass a breadth of topics, including European medical device regulations and vaccine management. By mastering the legal frameworks and ethical considerations, you'll be empowered to make informed decisions and contribute meaningfully to the healthcare landscape. Unlock the door to a world where healthcare, law, and ethics converge. Enrol in the Medical Law And Ethics Diploma today and embark on a transformative journey that will redefine your understanding of medical practices. This Medical Law And Ethics Diploma Bundle Consists of the following Premium courses: Course 01: Medical Law Course 02: European Medical Device Regulations Course 03: Medical Transcription Course 04: Control and Administration of Medicines Online Training Course Course 05: Consent in Healthcare Course 06: Health and Social Care Course 07: Care Planning and Record Keeping Course 08: Medical Terminology Training Course 09: English for Healthcare Course 10: Public Health Course 11: Vaccine Management The Medical Law And Ethics Diploma bundle offers an immersive learning experience designed to equip learners with the knowledge and skills required to navigate the legal and ethical landscape of healthcare in the UK. The bundle covers a breadth of topics, ranging from medical law and ethics to controlling and administering medicines. Through this comprehensive curriculum, learners will acquire the knowledge to make informed decisions and contribute to the healthcare landscape. Course Curriculum: Medical Law Module 01- An Introduction to Medical Law Module 02- Legislation on Access to Health, Medical Report, Treatment Module 03- Legislation on Adult Support Module 04- Legislation on Public Health and Health Service (Part 1) Module 05- Legislation on Public Health and Health Service (Part 2) Module 06- Legislation on Public Health and Health Service (Part 3) Module 07- Legislation on Public Health and Health Service (Part 4) Module 08- Legislation on Coronavirus Module 09- Legislation on Mental Health (Part 1) Module 10- Legislation on Mental Health (Part 2) Module 11- Legislation on Abortion Module 12- Other Legislation (Part 1) Module 13- Other Legislation (Part 2) ----------- 10 must have CPD Courses------------- CPD 110 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Medical Law And Ethics: Healthcare professionals seeking to deepen their knowledge of medical law and ethics. Law students interested in specialising in the healthcare sector. Individuals looking to pursue a career in healthcare administration. Professionals in the pharmaceutical industry seeking to expand their knowledge of medicine control and administration. Anyone seeking to gain a comprehensive understanding of the legal and ethical considerations involved in healthcare. Requirements This Medical Law And Ethics Diploma course has been designed to be fully compatible with tablets and smartphones. Career path Medical Law And Ethics: Healthcare Lawyer (£40,000 - £85,000) Medical Affairs Director (£75,000 - £150,000) Healthcare Compliance Officer (£28,000 - £60,000) Clinical Trial Manager (£30,000 - £75,000) Healthcare Policy Analyst (£28,000 - £55,000) Health and Safety Manager (£25,000 - £50,000) Healthcare Administrator (£18,000 - £35,000) Certificates Certificate of completion Digital certificate - Included Certificate of completion Hard copy certificate - £10 You will get the Hard Copy certificate for the Medical Law course absolutely Free! Other Hard Copy certificates are available for £10 each. Please Note: The delivery charge inside the UK is £3.99, and the international students must pay a £9.99 shipping cost.

Explore the intricacies of Clinical Research Administration with our comprehensive course on Protocols and Ethics. Gain expertise in trial design, ethical considerations, regulatory compliance, and more. Prepare for a future in clinical research with practical insights and knowledge. Enroll now for a rewarding journey in advancing healthcare through responsible and ethical clinical trials.

Search By Location
- Clinical Trial Administration Courses in London
- Clinical Trial Administration Courses in Birmingham
- Clinical Trial Administration Courses in Glasgow
- Clinical Trial Administration Courses in Liverpool
- Clinical Trial Administration Courses in Bristol
- Clinical Trial Administration Courses in Manchester
- Clinical Trial Administration Courses in Sheffield
- Clinical Trial Administration Courses in Leeds
- Clinical Trial Administration Courses in Edinburgh
- Clinical Trial Administration Courses in Leicester
- Clinical Trial Administration Courses in Coventry
- Clinical Trial Administration Courses in Bradford
- Clinical Trial Administration Courses in Cardiff
- Clinical Trial Administration Courses in Belfast
- Clinical Trial Administration Courses in Nottingham
