Booking options
£25
£25
On-Demand course
30 minutes
Intermediate level
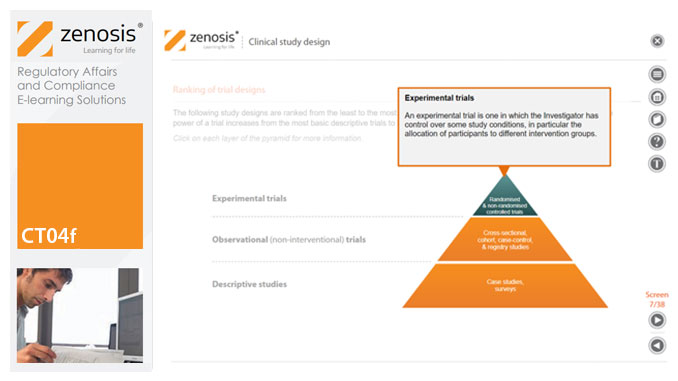
Clinical trial design establishes the framework upon which the clinical trial process will be conducted, and sets the objectives of the trial. The application for marketing approval, submitted to the regulatory authorities, will provide clinical data reflecting the trial design. Since trial design impacts the whole drug development process and lifecycle, particular care and due diligence is essential. This short course provides an overview of the main types of study design.
