- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
Courses in Leicester
We couldn't find any listings for your search. Explore our online options below.
Know someone teaching this? Help them become an Educator on Cademy.
Online Options
Show all 38Computer System Validation Training Course (ONLINE). Extend Your Role to CSV Projects. Get Certified and Become a CSV Professional
By Getreskilled (UK)
Has the Computer System Validation Engineer left and you’ve been handed their responsibilities? Do the thoughts of your next audit fill you with dread? CSV can be frustrating but this program will show you how to manage electronic data in a regulated manufacturing/laboratory/clinical environment using the GAMP framework and ensure compliance with FDA’s 21 CFR Part 11, EU Annex 11 or other regulatory guidelines.

CT04: An Introduction to Clinical Trial Preparation and Design
By Zenosis
This module aims to provide you with effective strategies for the preparation and conduct of a clinical trial, while adhering to regulatory safety standards. Management of data for submission is also covered.

CT08: Clinical Trial Monitoring: Study Monitoring, Documentation and Closure
By Zenosis
The sponsor of a clinical trial must arrange for it to be monitored throughout its duration to ensure that the rights and wellbeing of subjects are protected, the trial data are accurate, complete and verified from source documents, and the conduct of the trial complies with the study protocol, Good Clinical Practice and regulatory requirements. In this module we describe how a Clinical Research Associate (CRA) monitors an ongoing trial to its conclusion.
CT06: Clinical Trial Monitoring: Site Evaluation and Setup
By Zenosis
The sponsor of a clinical trial needs to reach agreement with clinical investigators to conduct the trial. The suitability of investigators and their institutional sites, typically hospitals, has to be evaluated, and the trial has to be set up at each site. This module describes the processes involved, focusing particularly on the role of a Clinical Research Associate (CRA) employed or contracted by the sponsor to monitor the trial.
***24 Hour Limited Time Flash Sale*** Clinical Trial Administrator (CTA) Admission Gifts FREE PDF & Hard Copy Certificate| PDF Transcripts| FREE Student ID| Assessment| Lifetime Access| Enrolment Letter Are you a professional feeling stuck in your career, struggling to keep up with the ever-changing demands of the industry? Or perhaps you're a beginner, unsure of where to start or how to break into your desired field. Whichever stage you're in, our exclusive Clinical Trial Administrator (CTA) Bundle provides unique insights and tools that can help you achieve your goals. Designed to cater to the needs of both seasoned professionals and aspiring newcomers, our Clinical Trial Administrator (CTA) bundle is a comprehensive program that will equip you with the essential skills and knowledge you need to succeed. Whether you're looking to advance in your current role or embark on a new career journey, this bundle has everything you need to take your professional life to the next level. But that's not all. When you enrol in Clinical Trial Administrator (CTA) Online Training, you'll receive 30 CPD-Accredited PDF Certificates, Hard Copy Certificates, and our exclusive student ID card, all absolutely free. Courses Are Included In this Clinical Trial Administrator (CTA) Career Bundle: Course 01: Medical & Clinical Administration Diploma Course 02: Project Management Course 03: Agile Project Management Course 04: Level 5 Diploma in Leadership and Management for Adult Care Course 05: Diploma in Operations Management Course 06: Clinical Governance Level 5 (endorsed by The Quality Licence Scheme) Course 07: Quality Control in Healthcare and Clinical Audit Course 08: Clinical Observations Skills Course 09: Clinical Psychology Course 10: An Introduction to Clinical Anaesthesia Course 11: Diploma in Clinical Coding (CPD Accredited) Course 12: Supervision in Adult Care Course 13: Research in Adult Care Course 14: Introduction to Medical Terminology Course 15: Anatomy and Physiology of the Human Body Course 16: Introduction to Health Economics and Health Technology Assessment Course 17: Initial Training of Community Health Agents Course 18: Medical Terminology: Course 19: Healthcare GDPR Training Course 20: Medical Secretary Diploma Course 21: Medical Receptionist Course Course 22: Medical Law Course 23: Medical Transcription Course 24: Nurse Prescribing Diploma Course 25: Pharmacy Assistant and Technician Foundation Diploma Course 26: Infection Prevention and Immunisation Course 27: Decision-Making in High-Stress Situations Course 28: Public Health Course 29: Effective Communication Course 30: Risk Assessment in Health & Social Care With Clinical Trial Administrator (CTA), you'll embark on an immersive learning experience that combines interactive lessons with voice-over audio, ensuring that you can learn from anywhere in the world, at your own pace. And with 24/7 tutor support, you'll never feel alone in your journey, whether you're a seasoned professional or a beginner. Don't let this opportunity pass you by. Enrol in Clinical Trial Administrator (CTA) today and take the first step towards achieving your goals and dreams. Why buy this Clinical Trial Administrator (CTA)? Free CPD Accredited Certificate upon completion of Clinical Trial Administrator (CTA) Get a free student ID card with Clinical Trial Administrator (CTA) Lifetime access to the Clinical Trial Administrator (CTA) course materials Get instant access to this Clinical Trial Administrator (CTA) course Learn Clinical Trial Administrator (CTA) from anywhere in the world 24/7 tutor support with the Clinical Trial Administrator (CTA) course. Start your learning journey straightaway with our Clinical Trial Administrator (CTA) Training! Clinical Trial Administrator (CTA) premium bundle consists of 30 precisely chosen courses on a wide range of topics essential for anyone looking to excel in this field. Each segment of Clinical Trial Administrator (CTA) is meticulously designed to maximise learning and engagement, blending interactive content and audio-visual modules for a truly immersive experience. Certification You have to complete the assignment given at the end of the Clinical Trial Administrator (CTA) course. After passing the Clinical Trial Administrator (CTA) exam You will be entitled to claim a PDF & Hardcopy certificate accredited by CPD Quality standards completely free. CPD 300 CPD hours / points Accredited by CPD Quality Standards Who is this course for? This Clinical Trial Administrator (CTA) course is ideal for: Students seeking mastery in Clinical Trial Administrator (CTA) Professionals seeking to enhance Clinical Trial Administrator (CTA) skills Individuals looking for a Clinical Trial Administrator (CTA)-related career. Anyone passionate about Clinical Trial Administrator (CTA) Requirements This Clinical Trial Administrator (CTA) doesn't require prior experience and is suitable for diverse learners. Career path Clinical Trial Manager Clinical Research Coordinator Clinical Operations Manager Clinical Project Manager Director of Clinical Operations Certificates CPD Accredited Digital certificate Digital certificate - Included CPD Accredited Hard copy certificate Hard copy certificate - Included If you are an international student, you will be required to pay an additional fee of 10 GBP for international delivery, and 4.99 GBP for delivery within the UK, for each certificate

CT04c - Clinical trial preparation
By Zenosis
The demands on quality from clinical trials are increasing. Quantitative aspects of clinical trials, such as the mass of study data to be collected, the multiple investigational sites, and the need to meet predetermined timelines, often supersede qualitative features. Therefore, addressing basic requirements for quality management is essential when preparing a clinical trial. This short course describes the core elements required for the establishment of a clinical trial and provides an overview of the role of the sponsor in supporting and improving trial quality.
CT03d - Clinical trial sponsor’s GCP responsibilities
By Zenosis
The sponsor of a clinical trial takes responsibility for its initiation, management, and/or financing. A sponsor may transfer any or all of the sponsor’s trial-related duties and functions to a contract research organisation, but the ultimate responsibility for the quality and integrity of the trial data always resides with the sponsor. Duties and functions discussed in this short course include trial design, selection of investigators, QA and QC, data handling and record keeping, finance and compensation, regulatory submissions, management of investigational product(s), safety reporting, monitoring, audit, dealing with noncompliance, and clinical trial reports. ICH guideline E6 (revision 2) encourages sponsors to adopt a risk-based approach to managing the quality of trials. We discuss this approach in general, and aspects such as risk-based monitoring in particular.
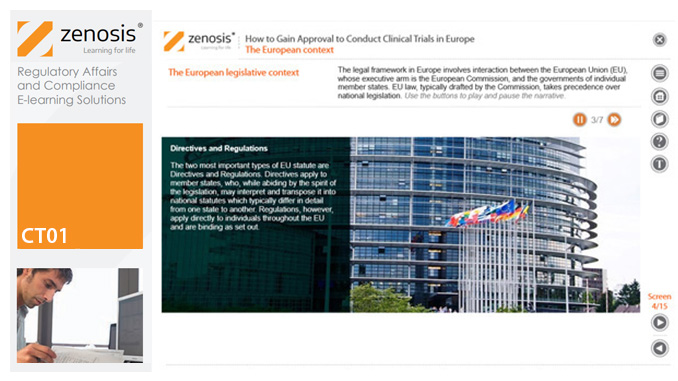
CT01: How to Gain and Maintain Approval for Clinical Research Under the EU Clinical Trials Directive
By Zenosis
To conduct a clinical trial in the European Economic Area under the Clinical Trials Directive the sponsor must apply for authorisation from the national competent authority (i.e. medicines regulator), and favourable opinion must be obtained from a research ethics committee, in each member state in which the trial is to take place. This module sets out the requirements for successful compilation, submission and maintenance of the applications.
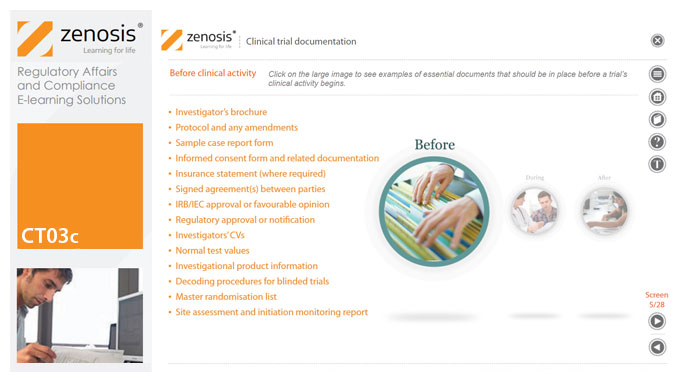
CT03c - Clinical trial documentation
By Zenosis
Regulatory authorities tend to abide by the maxim that ‘If it isn’t documented, it didn’t happen’. Rigorous documentation of all aspects of a clinical trial is necessary to provide evidence of GCP and compliance with regulatory requirements, as well as enabling effective management of the trial. In this short course we describe important examples of the documents designated by ICH GCP as essential to the conduct of a clinical trial.
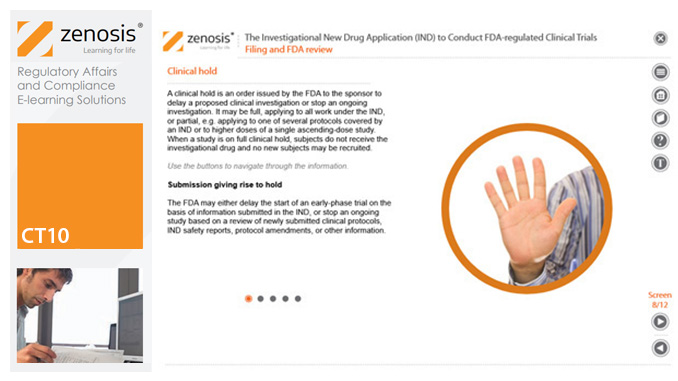
CT10: The Investigational New Drug Application (IND) to Conduct FDA-regulated Clinical Trials
By Zenosis
An Investigational New Drug Application (IND) is a submission to the US Food and Drug Administration (FDA) for permission to conduct a clinical trial of a medicinal product. This module describes regulatory requirements that sponsors or sponsor-investigators must meet for successful compilation, filing and maintenance of INDs. The IND and its role are defined, and the contexts in which it is required are specified.
Search By Location
- Clinical Trial Administration Courses in London
- Clinical Trial Administration Courses in Birmingham
- Clinical Trial Administration Courses in Glasgow
- Clinical Trial Administration Courses in Liverpool
- Clinical Trial Administration Courses in Bristol
- Clinical Trial Administration Courses in Manchester
- Clinical Trial Administration Courses in Sheffield
- Clinical Trial Administration Courses in Leeds
- Clinical Trial Administration Courses in Edinburgh
- Clinical Trial Administration Courses in Leicester
- Clinical Trial Administration Courses in Coventry
- Clinical Trial Administration Courses in Bradford
- Clinical Trial Administration Courses in Cardiff
- Clinical Trial Administration Courses in Belfast
- Clinical Trial Administration Courses in Nottingham