- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
1327 Courses
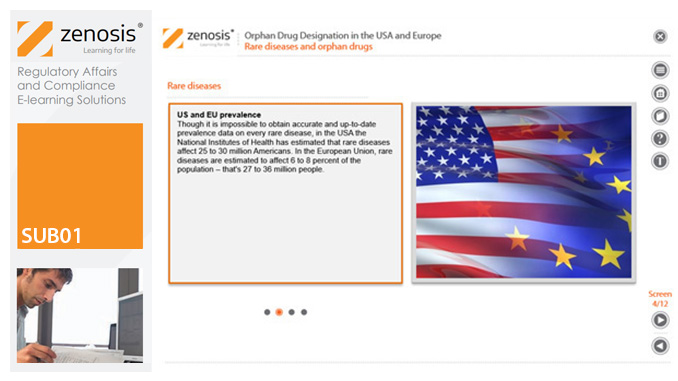
SUB01: Orphan Drug Designation in the USA and Europe
By Zenosis
Medicines for the prevention, diagnosis, or treatment of rare diseases have become known as ‘orphan drugs’ because of their commercial unattractiveness. Development of such products is successfully encouraged through incentives offered by regulatory authorities. To qualify for important incentives, the sponsor of a drug must gain ‘orphan designation’ for its use in an indication. This module describes the requirements for orphan designation and how to apply for it in the USA and the European Economic Area.
CT14: Clinical Trial Safety Reporting Requirements in the EU and USA
By Zenosis
This course sets out the legal and regulatory requirements for safety reporting in clinical trials of medicinal products under the jurisdictions of the European Union and the USA. It builds on the foundation laid by our companion course CT13, Safety Reporting in Clinical Trials, and provides greater detail of specific requirements in those jurisdictions.

Explore the intricate tapestry of Comparative Politics with our course on the UK and USA Comparative Systems. Uncover the foundations of government, analyze the US Constitution, dissect the roles of Congress, the President, and the Supreme Court. Dive into electoral processes and direct democracy while developing a keen understanding of comparative approaches. Gain valuable insights into the political landscapes of the United Kingdom and the United States, equipping yourself with the knowledge to critically assess and compare these dynamic systems. Enroll now to embark on a journey of political discovery and comparative analysis.

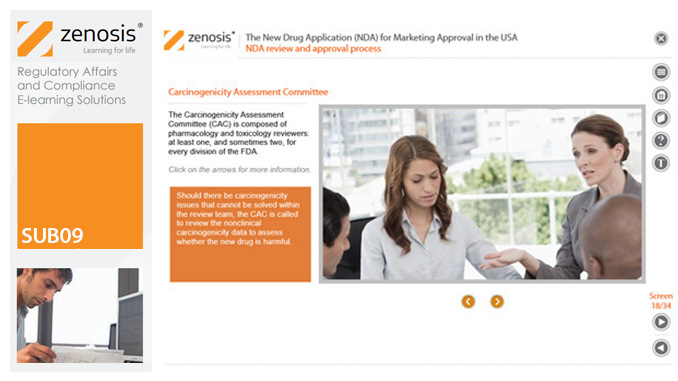
SUB09: The New Drug Application (NDA) for Marketing Approval in the USA
By Zenosis
The New Drug Application (NDA) is the regulatory vehicle through which sponsors formally propose that the Food and Drug Administration (FDA) approve a new pharmaceutical for marketing and sale in the USA.
Description: The Bookkeeping and Payroll USA Version - Video Training Course includes the essential issues related to Bookkeeping and Payroll. In the course, you will learn about the laws, rules, regulations, forms, records, and calculations of bookkeeping and payroll that a business owner or employee needs to run a business successfully. The course will help you to learn about the internal controls, subsidiary ledgers, reconciliations, sales tax, budgeting, accounting for merchandising and cash, partnerships and corporations, and cash flow. Apart from these, you will learn how to prepare a book for closing at the end of the years and the ways of developing interim statements. The course will teach you the complex payroll system simply so that you can apply your knowledge in the real world. Assessment: At the end of the course, you will be required to sit for an online MCQ test. Your test will be assessed automatically and immediately. You will instantly know whether you have been successful or not. Before sitting for your final exam you will have the opportunity to test your proficiency with a mock exam. Certification: After completing and passing the course successfully, you will be able to obtain an Accredited Certificate of Achievement. Certificates can be obtained either in hard copy at a cost of £39 or in PDF format at a cost of £24. Who is this Course for? Bookkeeping and Payroll USA Version - Video Training Course is certified by CPD Qualifications Standards and CiQ. This makes it perfect for anyone trying to learn potential professional skills. As there is no experience and qualification required for this course, it is available for all students from any academic background. Requirements Our Bookkeeping and Payroll USA Version - Video Training Course is fully compatible with any kind of device. Whether you are using Windows computer, Mac, smartphones or tablets, you will get the same experience while learning. Besides that, you will be able to access the course with any kind of internet connection from anywhere at any time without any kind of limitation. Career Path After completing this course you will be able to build up accurate knowledge and skills with proper confidence to enrich yourself and brighten up your career in the relevant job market. Principles Introduction - GAAP FREE 00:15:00 Transactions 00:11:00 Overview of Internal Controls The Need for Internal Controls 00:24:00 Control Concepts 00:25:00 Subsidiary Ledgers and Special Journals Subsidiary Ledgers 00:16:00 Special Journals - Posting Sales Journal 00:18:00 Special Journals - Posting Cash Receipts 00:13:00 Reconciliations Purpose 00:14:00 Reconciliation 00:22:00 Who Should Perform the Reconciliation 00:12:00 Correcting Entries Correcting Entries 00:08:00 The Wrong Account 00:05:00 Sales Tax Sales Tax 00:13:00 Sales Tax Rules and Filing 00:13:00 Budgeting All Businesses Must Have a Strategic Plan 00:28:00 Types of Budgets 00:19:00 Accounting for Merchandising Merchandising Income Statement 00:17:00 Sales Discounts 00:15:00 Purchase Discounts 00:17:00 Accounting for Cash Defining Petty Cash 00:12:00 Accounting for Cash Over or Short 00:13:00 Cash Controls - The Bank Reconciliation 00:27:00 Payroll The Payroll Process 00:35:00 Payroll Process - Deduction Tables 00:21:00 Payroll Process - Earnings Record 00:24:00 Partnerships and Corporations The Partnership 00:19:00 Corporations 00:18:00 Preferred Stock 00:18:00 Accounts Receivable and Bad Debts Accounts Receivable 00:22:00 Bad Debts 00:17:00 Interim Profit or Loss Interim 00:09:00 Year End - Preparing to Close the Books Inventory 00:21:00 Inventory Obsolescence 00:20:00 Year End - Closing the Books Year End - Closing Journal Entries 00:14:00 Year End - Post Closing Trial Balance 00:18:00 Cash Flow What is Cash Flow 00:19:00 Cash Flow - The Indirect Method 00:23:00 The Direct Method 00:22:00 Mock Exam Mock Exam- Bookkeeping and Payroll USA Version - Video Training Course 00:20:00 Final Exam Final Exam- Bookkeeping and Payroll USA Version - Video Training Course 00:20:00 Certificate and Transcript Order Your Certificates and Transcripts 00:00:00

Bookkeeping and Payroll USA Version Course is one of our best selling and most popular course. The Bookkeeping and Payroll USA Version Course is organised into 38 modules and includes everything you need to become successful in this profession. To make this course more accessible for you, we have designed it for both part-time and full-time students. You can study at your own pace or become an expert in just 12 hours! If you require support, our experienced tutors are always available to help you throughout the comprehensive syllabus of this course and answer all your queries through email. Why choose this course Earn an e-certificate upon successful completion. Accessible, informative modules taught by expert instructors Study in your own time, at your own pace, through your computer tablet or mobile device Benefit from instant feedback through mock exams and multiple-choice assessments Get 24/7 help or advice from our email and live chat teams Full Tutor Support on Weekdays Course Design The course is delivered through our online learning platform, accessible through any internet-connected device. There are no formal deadlines or teaching schedules, meaning you are free to study the course at your own pace. You are taught through a combination of Video lessons Online study materials Mock exams Multiple-choice assessment Certification After the successful completion of the final assessment, you will receive a CPD-accredited certificate of achievement. The PDF certificate is for £9.99, and it will be sent to you immediately after through e-mail. You can get the hard copy for £15.99, which will reach your doorsteps by post. Who is this course for? This Payroll Management course is ideal for professionals who work in a business or corporate setting, especially people who are assigned to manage accounts and payroll, as well as people who are interested in learning more about bookkeeping and payroll management. Course Content Principles Introduction - GAAP 00:15:00 Transactions 00:11:00 Overview of Internal Controls The Need for Internal Controls 00:24:00 Control Concepts 00:25:00 Subsidiary Ledgers and Special Journals Subsidiary Ledgers 00:16:00 Special Journals - Posting Sales Journal 00:18:00 Special Journals - Posting Cash Receipts 00:13:00 Reconciliations Purpose 00:14:00 Reconciliation 00:22:00 Who Should Perform the Reconciliation 00:12:00 Correcting Entries Correcting Entries 00:08:00 The Wrong Account 00:05:00 Sales Tax Sales Tax 00:13:00 Sales Tax Rules and Filing 00:13:00 Budgeting All Businesses Must Have a Strategic Plan 00:28:00 Types of Budgets 00:19:00 Accounting for Merchandising Merchandising Income Statement 00:17:00 Sales Discounts 00:15:00 Purchase Discounts 00:17:00 Accounting for Cash Defining Petty Cash 00:12:00 Accounting for Cash Over or Short 00:13:00 Cash Controls - The Bank Reconciliation 00:27:00 Payroll The Payroll Process 00:35:00 Payroll Process - Deduction Tables 00:21:00 Payroll Process - Earnings Record 00:24:00 Partnerships and Corporations The Partnership 00:19:00 Corporations 00:18:00 Preferred Stock 00:18:00 Accounts Receivable and Bad Debts Accounts Receivable 00:22:00 Bad Debts 00:17:00 Interim Profit or Loss Interim 00:09:00 Year End - Preparing to Close the Books Inventory 00:21:00 Inventory Obsolescence 00:20:00 Year End - Closing the Books Year End - Closing Journal Entries 00:14:00 Year End - Post Closing Trial Balance 00:18:00 Cash Flow What is Cash Flow 00:19:00 Cash Flow - The Indirect Method 00:23:00 The Direct Method 00:22:00 Mock Exam Mock Exam- Bookkeeping and Payroll USA Version 00:20:00 Final Exam Final Exam- Bookkeeping and Payroll USA Version 00:20:00 Order your Certificates & Transcripts Order your Certificates & Transcripts 00:00:00 Frequently Asked Questions Are there any prerequisites for taking the course? There are no specific prerequisites for this course, nor are there any formal entry requirements. All you need is an internet connection, a good understanding of English and a passion for learning for this course. Can I access the course at any time, or is there a set schedule? You have the flexibility to access the course at any time that suits your schedule. Our courses are self-paced, allowing you to study at your own pace and convenience. How long will I have access to the course? For this course, you will have access to the course materials for 1 year only. This means you can review the content as often as you like within the year, even after you've completed the course. However, if you buy Lifetime Access for the course, you will be able to access the course for a lifetime. Is there a certificate of completion provided after completing the course? Yes, upon successfully completing the course, you will receive a certificate of completion. This certificate can be a valuable addition to your professional portfolio and can be shared on your various social networks. Can I switch courses or get a refund if I'm not satisfied with the course? We want you to have a positive learning experience. If you're not satisfied with the course, you can request a course transfer or refund within 14 days of the initial purchase. How do I track my progress in the course? Our platform provides tracking tools and progress indicators for each course. You can monitor your progress, completed lessons, and assessments through your learner dashboard for the course. What if I have technical issues or difficulties with the course? If you encounter technical issues or content-related difficulties with the course, our support team is available to assist you. You can reach out to them for prompt resolution.

Project Economics, Risk and Decision Analysis for Oil & Gas
By EnergyEdge - Training for a Sustainable Energy Future
About this Training Course This 5 full-day training course looks at the setup of economic analysis cases, including the estimation of recoverable reserves, production profiles, commodity prices, and project costs - CAPEX, OPEX, taxes, royalties, transportation, depreciation, before-tax (BTAX) cash-flow, after-tax (ATAX) cash-flow and international fiscal regimes (production sharing agreement and concessionary system). The course begins from the basic required parameters of inflation, interest and time value of money. These concepts are then transformed into profitability indicators. Last but not the least, the profitability indicators are then used to make investment decisions. The emphasis of this course is to bridge the gap between theoretical concepts and their practical limitations. The participants will be able to appreciate the amount of information that they never thought of. Another emphasis of this course is also on the use of Excel's financial functions. This understanding is very critical when it comes to building economic cash-flow models. Over the years, we have seen that participants really struggle with using the Excel functions correctly and this leads to mistakes that can be easily avoided. In each session, multiple choice problems are provided to participants to reinforce their understanding of the concepts covered in the course. Many quick or tips not widely known, are also shared with the participants. The concepts covered in this course are not restricted to downstream, upstream or petrochemical projects. These concepts can be used to evaluate any type of investment under consideration. Participants will require access to computers/laptops with Excel to solve problems during the course. Training Objectives Upon completion of this course, participants will be able to fully understand the gas market dynamics and Investment Evaluation. They will be able to: Learn how to reduce exposure and mitigate risks in projects by handling uncertainty Clarify concepts such as time value of money, cash-flow models, capital budgeting, IRR, NPV, income producing investments Maximise the return on investments through good decision-making processes based on the commercial viability of projects Improve their decision process, investment and opportunity analysis Acquire the hands-on experience in building their own economic evaluation models and solving case study-based examples Target Audience The following oil & gas company personnel will benefit from the knowledge shared in this course: Facilities and Planning engineers Project and procurement personnel Oil & gas engineers Geologists Financial Analysts Commercial managers Economists Government officials Business advisors Asset managers E&P managers Product and business development personnel Course Level Basic or Foundation Training Methods Organisational Impact Trainer Your expert course leader is a globally recognised subject matter expert in petroleum/project economics and international gas market analysis. He is a recipient of the 2021 Society of Petroleum Engineers (SPE) International Management Award, an award that recognises individuals who make significant technical and professional contributions to the petroleum engineering profession and to the worldwide oil and gas industry. He has 40 years of diversified experience in petroleum engineering, reservoir engineering, project economics and decision analysis. He had been involved in evaluating multi-billion-dollar oil and gas field development, NGL, LNG, GTL, Aluminum smelter, refinery, petrochemical, power and production sharing projects. He has worked with major oil companies such as Saudi Aramco, ZADCO, Qatar Petroleum and companies in USA. He is a registered professional Engineer in the state of Colorado, USA. He is the author of six books: Petroleum Engineering Handbook for the Practicing Engineer, Vol. I and Vol. II, published by PennWell Books, Tulsa, Oklahoma, USA. Project Economics and Decision Analysis, Vol. I and Vol. II, published by PennWell Books, Tulsa, Oklahoma, USA. These books are used as textbooks in universities worldwide to teach petroleum economics to undergraduates and graduate students. Tip & Tricks in Excel based Financial Modeling, Vol. 1 & 2, published by Business Expert Press, New York, USA. He has also authored several papers in the Oil & Gas Journal, The Log Analyst, World Oil, SPE Journals, and Oil & Gas Financial Journal. He has delivered lectures in more than 25 countries around the globe. He has always received excellent feedback, as an expert presenter, from the participants of his courses. Daily daily_agenda POST TRAINING COACHING SUPPORT (OPTIONAL) To further optimise your learning experience from our courses, we also offer individualized 'One to One' coaching support for 2 hours post training. We can help improve your competence in your chosen area of interest, based on your learning needs and available hours. This is a great opportunity to improve your capability and confidence in a particular area of expertise. It will be delivered over a secure video conference call by one of our senior trainers. They will work with you to create a tailor-made coaching program that will help you achieve your goals faster. Request for further information post training support and fees applicable Accreditions And Affliations

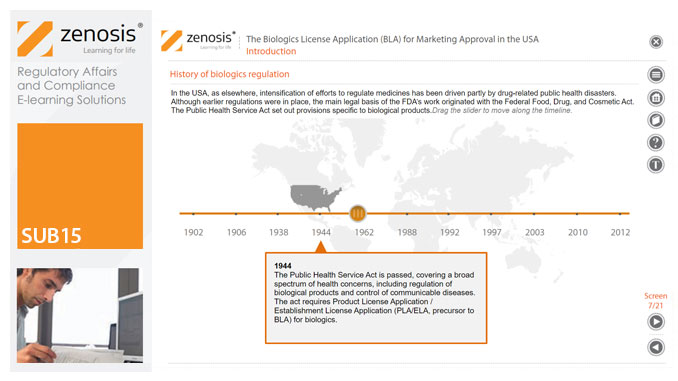
SUB15: The Biologics License Application (BLA) for Marketing Approval in the USA
By Zenosis
This module describes the requirements that must be met to obtain licensure of a biological product. Subjects covered include the regulatory context, the content and format of the BLA submission, the review process, and provisions for expedited development and review.
SAM04: Marketing of Prescription Drugs in the USA – Interactions with Healthcare Professionals
By Zenosis
The heaviest legal penalties imposed on drug companies concern interactions with healthcare professionals in the context of prescription drug marketing, notably for violations of the Anti-Kickback Statute and the False Claims Act. Monetary penalties have amounted to billions of dollars in some cases.

SAM02: Regulatory Requirements and Guidance on Advertising and Promotion of Prescription Drugs in the USA
By Zenosis
In this course we explain how to advertise and promote prescription drugs in various media, whether to healthcare professionals or consumers, in compliance with legal requirements and guidance from the FDA.

Search By Location
- usa Courses in London
- usa Courses in Birmingham
- usa Courses in Glasgow
- usa Courses in Liverpool
- usa Courses in Bristol
- usa Courses in Manchester
- usa Courses in Sheffield
- usa Courses in Leeds
- usa Courses in Edinburgh
- usa Courses in Leicester
- usa Courses in Coventry
- usa Courses in Bradford
- usa Courses in Cardiff
- usa Courses in Belfast
- usa Courses in Nottingham